This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, key market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the sepsis diagnostics market. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in the sepsis diagnostics market. The primary sources from the demand side include OEMs, private and contract testing organizations and service providers, among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
A breakdown of the primary respondents is provided below:
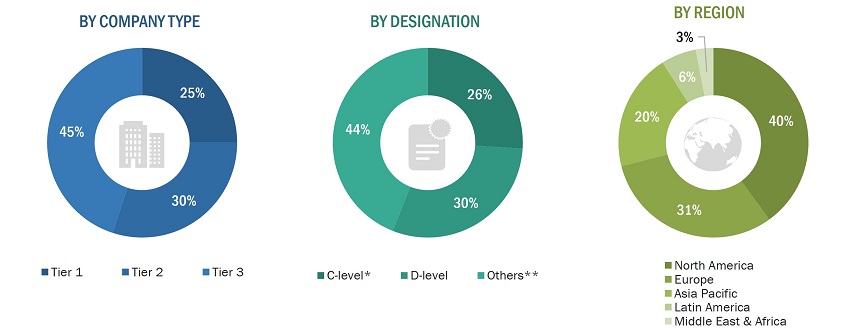
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation Methodology
In this report, the global sepsis diagnostics market size was arrived at by using the revenue share analysis of leading players. For this purpose, key players in the market were identified, and their revenues from the sepsis diagnostics products business were determined through various insights gathered during the primary and secondary research phases. Secondary research included the study of the annual and financial reports of the top market players. In contrast, primary research included extensive interviews with key opinion leaders, such as CEOs, directors, and key marketing executives.
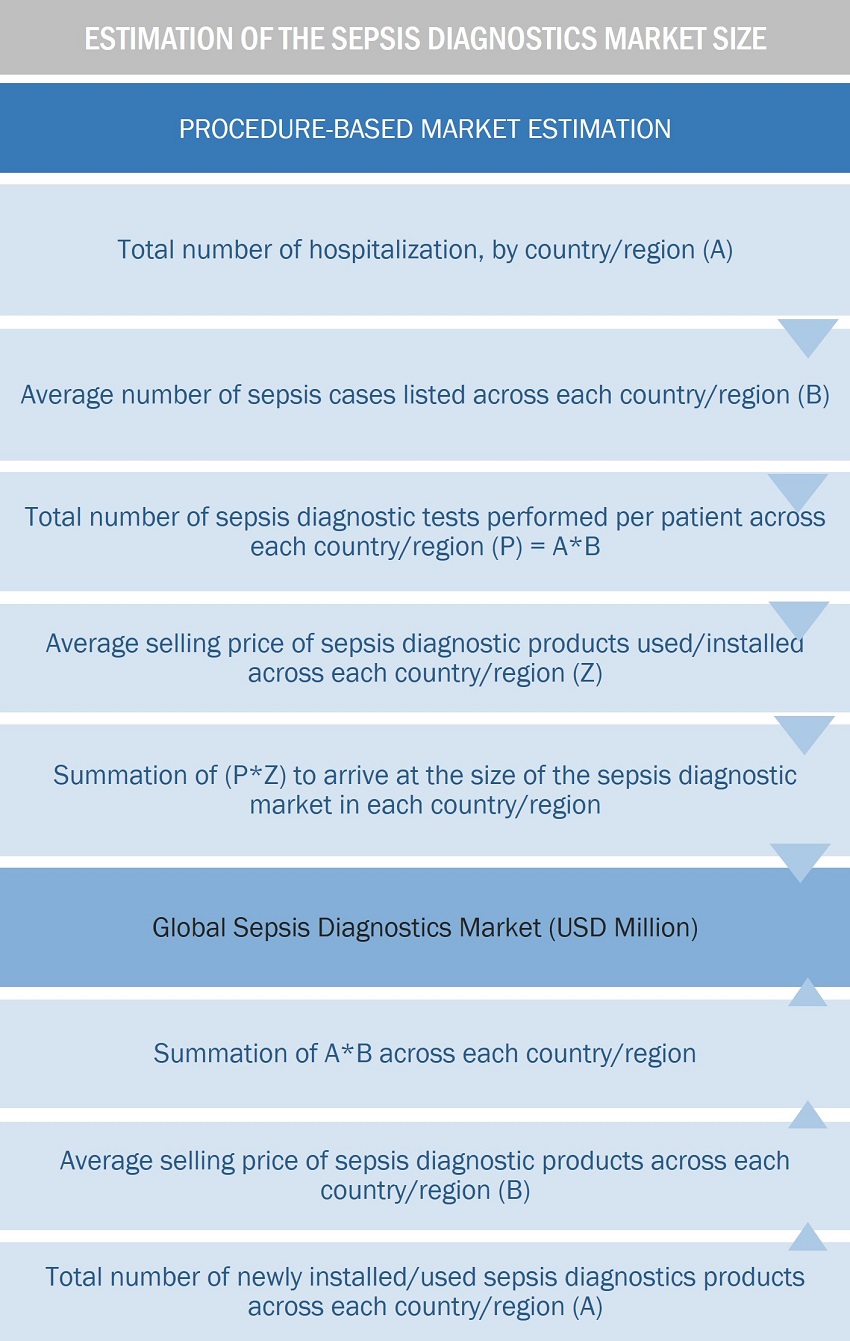
Approach 1: Procedure-based estimation approach
To calculate the global market value, installation/volume consumption of sepsis diagnostic products were calculated. This process involved the following steps:
-
Finding the total number of hospitalization at the regional and/or country-level
-
The average number of sepsis cases at the regional and/or country level
-
The average number of sepsis diagnostics tests performed per patient at the regional and/or country-level
-
The average selling price of each product type for a given year was triangulated to arrive at the market value data at the regional and/or country-level
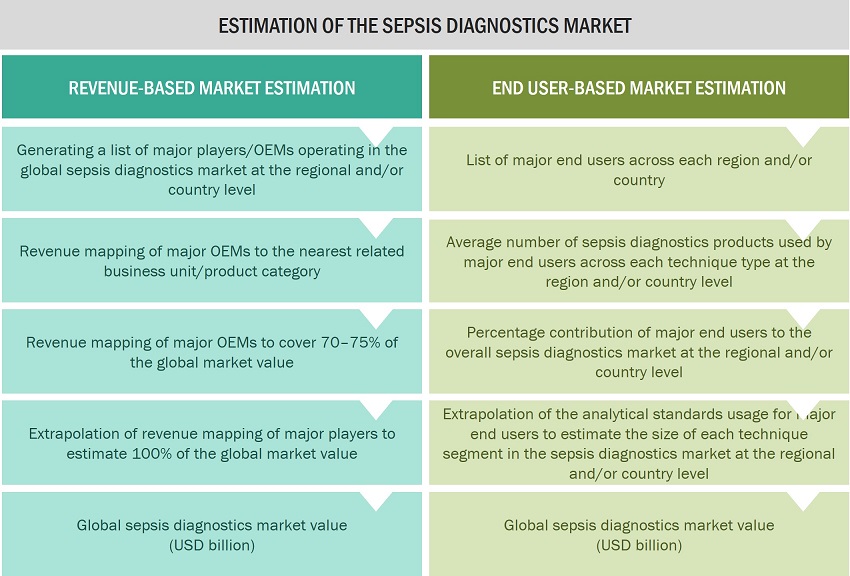
Approach 2: End user-based market estimation
During preliminary secondary research, the total sales revenue of sepsis diagnostics was estimated and validated at the regional and country level, triangulated, and validated to estimate the global market value. This process involved the following steps:
-
Generating a list of major customer facilities across each region and country
-
Identifying the average number of sepsis diagnostics product supplies used by major customer facilities across each product type at the regional/country level, annually
-
Identifying the percentage contribution of major customer facilities to the overall sepsis diagnostics expenditure and usage at the regional/country level, annually
-
Extrapolating the annual usage patterns for various products across major customer facilities to estimate the size of each product segment at the regional/country level, annually
To know about the assumptions considered for the study, Request for Free Sample Report
Data Triangulation
After arriving at the overall market size from the market size estimation process explained above, the global sepsis diagnostics market was split into segments and subsegments. Data triangulation and market breakdown procedures were employed to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Additionally, the sepsis diagnostics market was validated using both top-down and bottom-up approaches.
Market Definition
Sepsis is a life-threatening condition caused by pathogen infection. It develops when chemicals released into the bloodstream to fight the infection trigger inflammatory responses throughout the body. This is a three-stage syndrome—from sepsis to severe sepsis and septic shock. Septic shock is the last stage that results in extremely low blood pressure and a lack of response to normal fluid replacement. Inflammation leads to multi-organ failure; progress to septic shock could lead to death.
Key Market Stakeholders
-
Sepsis diagnostics reagents, instruments, blood culture media manufacturers
-
Research & academic laboratories
-
Healthcare service providers (including hospitals, reference labs, diagnostic centers, and specialty clinics)
-
Market research and consulting firms
-
Medical device suppliers, distributors, channel partners, and third-party suppliers
-
Business research and consulting service providers
-
Venture capitalists and other government funding organizations
Objectives of the Study
-
To define, describe, and forecast the sepsis diagnostics market on the basis of on technology, product, method, pathogen type, test type, end user and region.
-
To provide detailed information regarding the major factors influencing the growth potential of the global sepsis diagnostics market (drivers, restraints, opportunities, challenges, and trends).
-
To analyze the micro markets with respect to individual growth trends, future prospects, and contributions to the global sepsis diagnostics market.
-
To analyze key growth opportunities in the global sepsis diagnostics market for key stakeholders and provide details of the competitive landscape for market leaders.
-
To forecast the size of market segments and/or subsegments with respect to five major regions, namely, North America (the US and Canada), Europe (Germany, the UK, France, Italy, Spain, and Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, and Rest of Asia Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East and Africa (GCC Countries and Rest of MEA).
-
To profile the key players in the global sepsis diagnostics market and comprehensively analyze their market shares and core competencies.
-
To track and analyze the competitive developments undertaken in the global sepsis diagnostics market, such as agreements, expansions, and & acquisitions.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the present global sepsis diagnostics market report:
Product Analysis
-
Product matrix, which gives a detailed comparison of the product portfolios of the top fifteen companies
Company Information
-
Detailed analysis and profiling of additional market players (up to 15)
Geographic Analysis
-
Further breakdown of the Rest of Europe's sepsis diagnostics market into Russia, Belgium, the Netherlands, Switzerland, Austria, Finland, Sweden, Poland, and Portugal among other
-
Further breakdown of the Rest of Asia Pacific sepsis diagnostics market into Singapore, Taiwan, New Zealand, Philippines, Malaysia, and other APAC countries
-
Further breakdown of the Rest of the Latin America sepsis diagnostics market into Argentina, Chile, Peru, and Colombia, among other



catherine
May, 2020
Have you not heard of T2 Biosystems rapid diagnostics direct from blood? Much faster than blood cultures, which is vital considering how quickly sepsis kills. FDA and CMS labeled it "breakthrough technology" and CMS subsidizes the test at 65, $97..
Barbara
Mar, 2022
What are the after-effects of the COVID19 on the Global Sepsis Diagnostics Industry?.
Susan
Mar, 2022
What are the upcoming trends in the Global Sepsis Diagnostics Industry?.