2
RESEARCH METHODOLOGY
40
5
MARKET OVERVIEW
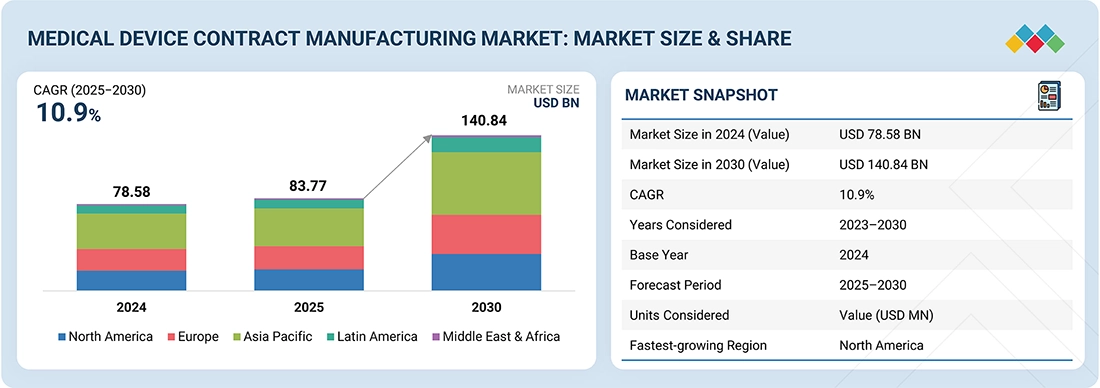
Emerging markets drive growth as automation and AI reshape the medical device landscape.
62
5.2.1.1
GROWING MEDICAL DEVICES MARKET IN DEVELOPING COUNTRIES
5.2.1.2
ADOPTION OF ROBOTICS AND AUTOMATION IN MANUFACTURING
5.2.1.3
TECHNOLOGICAL ADVANCEMENTS IN MEDICAL DEVICE MODALITIES
5.2.1.4
IMPACT OF INDUSTRY 4.0 ON MEDICAL DEVICE INDUSTRY
5.2.2.1
CONSOLIDATION IN MEDICAL DEVICES MARKET
5.2.3.1
INCREASING HEALTHCARE EXPENDITURE, INFRASTRUCTURE, AND AWARENESS IN DEVELOPING ECONOMIES
5.2.3.2
RISING GERIATRIC POPULATION AND INCREASING INCIDENCE OF ASSOCIATED DISEASES
5.2.4.1
LACK OF CONSTANT INNOVATION TO BALANCE TECHNOLOGICAL CAPABILITIES AGAINST COSTS
5.3.1
INCREASING CONSOLIDATION OF OEMS AND CONTRACT MANUFACTURERS
5.3.2
GROWING INTEREST OF PRIVATE EQUITY FIRMS IN MEDICAL DEVICE CONTRACT MANUFACTURING
5.3.3
OUTSOURCING OF MEDICAL DEVICE CONTRACT MANUFACTURING SERVICES
5.4.1.1
DIGITAL TRANSFORMATION
5.4.1.2
ROBOTICS AND AUTOMATION
5.4.2
ADJACENT TECHNOLOGIES
5.4.2.1
ARTIFICIAL INTELLIGENCE
5.4.3
COMPLEMENTARY TECHNOLOGIES
5.5
PORTER’S FIVE FORCES ANALYSIS
5.5.1
THREAT OF NEW ENTRANTS
5.5.2
THREAT OF SUBSTITUTES
5.5.3
BARGAINING POWER OF SUPPLIERS
5.5.4
BARGAINING POWER OF BUYERS
5.5.5
INTENSITY OF COMPETITIVE RIVALRY
5.6
KEY STAKEHOLDERS AND BUYING CRITERIA
5.6.1
KEY STAKEHOLDERS IN BUYING PROCESS
5.7.1
REGULATORY FRAMEWORK
5.7.2
REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.8.1
AVERAGE SELLING PRICE TREND, BY KEY PLAYER, 2022–2024
5.8.2
AVERAGE SELLING PRICE TREND, BY REGION, 2022–2024
5.10
KEY CONFERENCES AND EVENTS IN 2025–2026
5.11.1
LIST OF MAJOR PATENTS, 2022–2025
5.12
SUPPLY CHAIN ANALYSIS
5.14.1
IMPORT DATA FOR HS CODE 9018, 2020–2024
5.14.2
EXPORT DATA FOR HS CODE 9018, 2020–2024
5.15
ADJACENT MARKETS FOR MEDICAL DEVICE CONTRACT MANUFACTURING
5.16
UNMET NEEDS/END-USER EXPECTATIONS IN MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
5.17
IMPACT OF GEN AI IN MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
5.18.1
CASE STUDY 1: JABIL INC. AND HEARTWARE INTERNATIONAL PARTNER TO DEVELOP ADVANCED HEART FAILURE TREATMENT
5.18.2
CASE STUDY 2: FLEX LTD. AND MEDTRONIC COLLABORATE TO DEVELOP THE NEXT-GENERATION INSULIN PUMP
5.18.3
CASE STUDY 3: PLEXUS PARTNERED WITH A START-UP TO DEVELOP A REVOLUTIONARY INSULIN PUMP
5.19
INVESTMENT AND FUNDING SCENARIO
5.20
IMPACT OF 2025 US TARIFFS—MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
5.20.3
PRICE IMPACT ANALYSIS
5.20.4
IMPACT ON COUNTRIES/REGIONS
5.20.5
IMPACT ON END-USE INDUSTRIES
5.20.5.1
DIAGNOSTIC & IMAGING EQUIPMENT
5.20.5.2
SURGICAL INSTRUMENTS & IMPLANTS
5.20.5.3
POINT-OF-CARE & DIAGNOSTIC KITS
6
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE
Market Size & Growth Rate Forecast Analysis to 2035 in USD Million | 31 Data Tables
104
6.2.1.1
INCREASING DEMAND FOR REAGENTS & KITS TO DRIVE MARKET
6.2.2.1
ADVANCES IN LIFE SCIENCES RESEARCH TO SUPPORT MARKET
6.3
DIAGNOSTIC IMAGING DEVICES
6.3.1
ADOPTION OF NEW AND ADVANCED DIAGNOSTIC IMAGING SYSTEMS IN DEVELOPING COUNTRIES TO AUGMENT MARKET
6.4
CARDIOVASCULAR DEVICES
6.4.1
RISING PREVALENCE OF CVDS TO PROPEL MARKET
6.5
DRUG DELIVERY DEVICES
6.5.1
INFUSION DEVICES & ADMINISTRATION SETS
6.5.1.1
RISING INCIDENCE OF CHRONIC DISEASES AND NUMBER OF SURGICAL PROCEDURES TO FUEL MARKET
6.5.2.1
HIGH COST OF SAFETY SYRINGES AND INCREASING INCIDENCE OF NEEDLESTICK INJURIES TO HINDER MARKET
6.5.3.1
HIGH PREVALENCE OF ASTHMA, COPD, AND CYSTIC FIBROSIS TO AID MARKET GROWTH
6.5.4.1
RISING NUMBER OF REGULATORY APPROVALS FOR NEW DRUG THERAPIES AND ADVANCEMENTS TO DRIVE MARKET
6.5.5.1
RISING PREVALENCE OF CHRONIC DISEASES AND GROWING PIPELINE OF BIOLOGICS AND BIOSIMILARS TO BOOST MARKET GROWTH
6.5.6
OTHER DRUG DELIVERY DEVICES
6.6.1
HIGH PREVALENCE OF ORTHOPEDIC CONDITIONS TO DRIVE MARKET
6.7
RESPIRATORY CARE DEVICES
6.7.1
GROWING PREVALENCE OF RESPIRATORY INFECTIONS TO DRIVE MARKET
6.8
OPHTHALMOLOGY DEVICES
6.8.1
INCREASING PREVALENCE OF OPHTHALMIC DISEASES TO DRIVE MARKET
6.9.1
INCREASE IN SURGICAL PROCEDURES TO FUEL MARKET
6.10
DIABETES CARE DEVICES
6.10.1
RISING PREVALENCE OF DIABETES TO PROPEL MARKET
6.11.1
RISING INCIDENCE OF DENTAL DISEASES TO DRIVE MARKET
6.12
ENDOSCOPY & LAPAROSCOPY DEVICES
6.12.1
RISING PATIENT PREFERENCE FOR MINIMALLY INVASIVE PROCEDURES TO AUGMENT MARKET
6.13
GYNECOLOGY/UROLOGY DEVICES
6.13.1
GROWING AWARENESS AND PREVENTIVE CHECK-UPS FOR LATE-PHASE DIAGNOSIS OF STDS TO PROPEL MARKET
6.14
PERSONAL CARE DEVICES
6.14.1
SHIFT IN CONSUMPTION PATTERN TOWARD PREMIUM PERSONAL CARE PRODUCTS TO FUEL MARKET
6.15.1
RISING GLOBAL BURDEN OF NEUROLOGICAL DISEASES TO DRIVE MARKET
6.16
PATIENT MONITORING DEVICES
6.16.1
GROWING AVAILABILITY OF INTEGRATED MONITORING TECHNOLOGY TO AID MARKET
6.17
PATIENT ASSISTIVE DEVICES
6.17.1
RISING GERIATRIC POPULATION TO PROPEL MARKET
7
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 4 Data Tables
157
7.2
CLASS II MEDICAL DEVICES
7.2.1
HIGH-VOLUME UTILIZATION OF CLASS II MEDICAL DEVICES TO SUPPORT MARKET
7.3
CLASS I MEDICAL DEVICES
7.3.1
CLASS I DEVICES HOLD LARGEST SHARE OF ALL APPROVED MEDICAL DEVICES
7.4
CLASS III MEDICAL DEVICES
7.4.1
HIGHEST LEVEL OF HEALTH RISKS NECESSITATES STRINGENT OVERSIGHT
8
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 18 Data Tables
164
8.2
DEVICE DEVELOPMENT & MANUFACTURING SERVICES
8.2.1
DEVICE & COMPONENT MANUFACTURING SERVICES
8.2.1.1
NEW ASPECTS, SUCH AS ROBOTICS AND PERSONALIZED MEDICINE, TO NECESSITATE SPECIALIZED DEVICE MANUFACTURING CAPABILITIES
8.2.2
PROCESS DEVELOPMENT SERVICES
8.2.2.1
INCREASED R&D SPENDING AND INNOVATION AND GROWING DEMAND FOR COMPLEX PRODUCTS TO DRIVE MARKET
8.2.3
DEVICE ENGINEERING SERVICES
8.2.3.1
COMPLEXITY OF DEVICE ENGINEERING SERVICES TO PROPEL SEGMENT
8.3
QUALITY MANAGEMENT SERVICES
8.3.1
PACKAGING VALIDATION SERVICES
8.3.1.1
INCREASING PRODUCT SAFETY CONCERNS FOR MEDICAL DEVICES TO AID MARKET
8.3.2
INSPECTION & TESTING SERVICES
8.3.2.1
AVAILABILITY OF TECHNOLOGICAL EXPERTISE IN TESTING SERVICES TO ELIMINATE LARGE CAPITAL AND OVERHEAD INVESTMENTS
8.3.3
STERILIZATION SERVICES
8.3.3.1
RISING COMPLEXITY OF STERILITY STANDARDS TO AUGMENT MARKET
8.4
PACKAGING & ASSEMBLY SERVICES
8.4.1
PRIMARY & SECONDARY PACKAGING SERVICES
8.4.1.1
GROWING CONCERNS FOR PATIENT SAFETY TO AID SEGMENT
8.4.2.1
EXPORT BANS AND PRODUCT RECALLS DUE TO IMPROPER LABELING TO SUPPORT MARKET
8.4.3
OTHER PACKAGING & ASSEMBLY SERVICES
9
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY REGION
Comprehensive coverage of 9 Regions with country-level deep-dive of 21 Countries | 282 Data Tables.
191
9.2.1
MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
9.2.2.1
LOW LABOR COSTS AND RAPIDLY CHANGING HEALTHCARE INFRASTRUCTURE TO DRIVE MARKET
9.2.3.1
RISING CONTRACT MANUFACTURING CAPABILITIES TO SUPPORT MARKET GROWTH
9.2.4.1
UNIVERSAL HEALTHCARE REIMBURSEMENT POLICIES TO PROPEL MARKET
9.2.5.1
INCREASING R&D ACTIVITIES AND INVESTMENTS BY ESTABLISHED PLAYERS TO AUGMENT MARKET GROWTH
9.2.6.1
GOVERNMENT-SUPPORTED INDUSTRIAL CLUSTERING AND EXPORT COMPETITIVENESS TO PROPEL MARKET
9.2.7.1
HIGH-VALUE IMPORTS FROM OTHER COUNTRIES TO OFFER GROWTH OPPORTUNITIES
9.2.8.1
GOVERNMENT-BACKED INVESTMENT IN SOVEREIGN CAPABILITY TO DRIVE MEDTECH MANUFACTURING GROWTH
9.2.9.1
SKILLED LABOR FORCE AND IT-ENABLED SUPPORT TO DRIVE MARKET GROWTH
9.2.10.1
STRONG GOVERNMENT SUPPORT AND GLOBAL INVESTMENTS TO DRIVE MARKET
9.2.11.1
DEREGULATION OF THERAPEUTIC PRODUCTS UNLOCKING EXPEDITED ACCESS AND EXPORT POTENTIAL
9.2.12
REST OF ASIA PACIFIC
9.3.1
MACROECONOMIC OUTLOOK FOR EUROPE
9.3.2.1
PRESENCE OF MAJOR DEVICE MANUFACTURERS AND HIGH HEALTHCARE EXPENDITURE TO SUPPORT MARKET GROWTH
9.3.3.1
RISING NUMBER OF ACCREDITED CLINICAL LABORATORIES AND HOSPITAL LABORATORIES TO PROPEL MARKET
9.3.4.1
INCREASING GOVERNMENT PRESSURE FOR HEALTHCARE COST REDUCTION AND BUDGETARY CONSTRAINTS TO SUPPORT MARKET GROWTH
9.3.5.1
INCREASING ADOPTION OF HOME-BASED MEDICAL DEVICES TO DRIVE MARKET
9.3.6.1
HEALTHCARE COVERAGE FOR ALL CITIZENS AND FOREIGN RESIDENTS TO INCREASE DEMAND FOR MEDICAL DEVICES
9.4.1
MACROECONOMIC OUTLOOK FOR NORTH AMERICA
9.4.2.1
PRESENCE OF STRINGENT GOVERNMENT REGULATIONS TO DRIVE MARKET
9.4.3.1
STRONG PRESENCE OF KEY CONTRACT MANUFACTURING COMPANIES TO AUGMENT MARKET
9.5.1
MACROECONOMIC OUTLOOK FOR LATIN AMERICA
9.5.2.1
FAVORABLE GOVERNMENT INITIATIVES AND WILLINGNESS TO PAY FOR BETTER HEALTHCARE TO BOOST MARKET
9.5.3.1
PROXIMITY TO US AND OTHER LATAM COUNTRIES TO STRENGTHEN MEXICO’S RELEVANCE AS A CONTRACT MANUFACTURING HUB
9.5.4
REST OF LATIN AMERICA
9.6.1
MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
9.6.2.1
INCREASING HEALTHCARE INFRASTRUCTURE DEVELOPMENT TO SUPPORT MARKET GROWTH
9.6.2.2
KINGDOM OF SAUDI ARABIA (KSA)
9.6.2.3
UNITED ARAB EMIRATES (UAE)
9.6.2.4
REST OF GCC COUNTRIES
9.6.3
REST OF MIDDLE EAST AND AFRICA
10
COMPETITIVE LANDSCAPE
Discover emerging leaders and strategic shifts shaping the medical device contract manufacturing market.
326
10.1.1
KEY STRATEGIES ADOPTED BY PLAYERS IN MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
10.2
REVENUE ANALYSIS, 2020−2024
10.3
MARKET SHARE ANALYSIS, 2024
10.4
COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
10.4.5
COMPANY FOOTPRINT: KEY PLAYERS, 2024
10.4.5.1
COMPANY FOOTPRINT
10.4.5.2
REGION FOOTPRINT
10.4.5.3
DEVICE FOOTPRINT
10.4.5.4
DEVICE CLASS FOOTPRINT
10.4.5.5
SERVICE FOOTPRINT
10.5
COMPANY EVALUATION MATRIX, STARTUPS/SMES, 2024
10.5.1
PROGRESSIVE COMPANIES
10.5.2
RESPONSIVE COMPANIES
10.5.5
COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
10.5.5.1
DETAILED LIST OF KEY STARTUPS/SMES
10.5.5.2
COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
10.6
COMPANY VALUATION & FINANCIAL METRICS
10.7
BRAND/PRODUCT COMPARATIVE ANALYSIS
10.8
R&D ASSESSMENT OF KEY PLAYERS
10.9
COMPETITIVE SCENARIO
11
COMPANY PROFILES
In-depth Company Profiles of Leading Market Players with detailed Business Overview, Product and Service Portfolio, Recent Developments, and Unique Analyst Perspective (MnM View)
346
11.1.1.1
BUSINESS OVERVIEW
11.1.1.2
PRODUCTS/SERVICES/SOLUTIONS OFFERED
11.1.1.3
RECENT DEVELOPMENTS
11.1.4
SANMINA CORPORATION
11.1.5
INTEGER HOLDINGS CORPORATION
11.1.6
TE CONNECTIVITY LTD.
11.1.9
WEST PHARMACEUTICAL SERVICES, INC.
11.1.10
BENCHMARK ELECTRONICS INC.
11.1.13
KIMBALL ELECTRONICS, INC.
11.1.14
NORTECH SYSTEMS, INC.
11.1.16
NOLATO GW, INC. (A PART OF NOLATO AB)
11.2.2
VIANT MEDICAL HOLDINGS, INC.
11.2.5
PHILLIPS-MEDISIZE CORPORATION
11.2.6
TESSY PLASTICS CORP.
11.2.9
PETER’S TECHNOLOGY
12.2
KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
12.3
CUSTOMIZATION OPTIONS
TABLE 1
STANDARD CURRENCY CONVERSION RATES (UNIT OF USD)
TABLE 2
RISK ASSESSMENT: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
TABLE 3
KEY ACQUISITIONS IN MEDICAL DEVICE INDUSTRY
TABLE 4
ESTIMATED INCREASE IN GERIATRIC POPULATION, BY REGION, 2019 VS. 2050
TABLE 5
AVERAGE LIFE EXPECTANCY, 2023
TABLE 6
KEY EXAMPLES OF OEM CONSOLIDATION
TABLE 7
KEY EXAMPLES OF CMO CONSOLIDATION
TABLE 8
KEY INVESTMENTS BY PRIVATE EQUITY FIRMS
TABLE 9
EXAMPLES OF KEY COLLABORATIONS AND AGREEMENTS
TABLE 10
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: PORTER’S FIVE FORCES ANALYSIS
TABLE 11
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR MEDICAL DEVICES
TABLE 12
KEY BUYING CRITERIA FOR MEDICAL DEVICES
TABLE 13
US: CLASSIFICATION OF MEDICAL EQUIPMENT
TABLE 14
US: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 15
CANADA: RISK, TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 16
EUROPE: RISK, TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 17
JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 18
CHINA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 19
NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 20
EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 21
ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 22
LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 23
MIDDLE EAST & AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 24
AVERAGE SELLING PRICE TREND OF MEDICAL DEVICE CONTRACT MANUFACTURING SERVICES, BY DEVICE, 2022–2024 (USD)
TABLE 25
AVERAGE SELLING PRICE TREND OF MEDICAL DEVICE CONTRACT MANUFACTURING SERVICES, BY REGION, 2022–2024 (USD)
TABLE 26
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DETAILED LIST OF CONFERENCES AND EVENTS
TABLE 27
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: LIST OF MAJOR PATENTS, JANUARY 2022–JUNE 2025
TABLE 28
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: ROLE IN ECOSYSTEM, 2024
TABLE 29
IMPORT DATA FOR HS CODE 9018, BY COUNTRY, 2020–2024 (USD BILLION)
TABLE 30
EXPORT DATA FOR HS CODE 9018, BY COUNTRY, 2020–2024 (USD BILLION)
TABLE 31
US ADJUSTED RECIPROCAL TARIFF RATES
TABLE 32
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 33
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 34
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 35
IVD CONSUMABLES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 36
IVD EQUIPMENT MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 37
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DIAGNOSTIC IMAGING DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 38
PREVALENCE OF CVDS, 2015–2035
TABLE 39
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR CARDIOVASCULAR DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 40
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 41
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 42
INFUSION DEVICES & ADMINISTRATION SETS MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 43
SYRINGES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 44
INHALERS MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 45
RECENT APPROVALS FOR AUTOINJECTORS AND PEN INJECTORS GLOBALLY
TABLE 46
AUTOINJECTORS MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 47
PEN INJECTORS MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 48
OTHER DRUG DELIVERY DEVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 49
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR ORTHOPEDIC DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 50
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR RESPIRATORY CARE DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 51
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR OPHTHALMOLOGY DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 53
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR SURGICAL DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 54
TOP FIVE COUNTRIES WITH HIGHEST NUMBER OF DIABETIC PATIENTS (20-79 YRS) (2024 V/S 2050)
TABLE 55
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DIABETES CARE DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 57
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DENTAL DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 59
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR ENDOSCOPY & LAPAROSCOPY DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 61
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR GYNECOLOGY/UROLOGY DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 63
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR PERSONAL CARE DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 64
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR NEUROLOGY DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 65
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR PATIENT MONITORING DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 66
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR PATIENT ASSISTIVE DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 67
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR OTHER DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 68
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 69
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR CLASS II MEDICAL DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 70
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR CLASS I MEDICAL DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 72
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR CLASS III MEDICAL DEVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 73
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 74
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DEVICE DEVELOPMENT & MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 75
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DEVICE DEVELOPMENT & MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 77
DEVICE & COMPONENT MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 78
PROCESS DEVELOPMENT SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 79
DEVICE ENGINEERING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 80
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR QUALITY MANAGEMENT SERVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 81
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR QUALITY MANAGEMENT SERVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 82
PACKAGING VALIDATION SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 83
INSPECTION & TESTING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 84
MEDICAL DEVICE STERILIZATION PATENTS PUBLISHED BY MAJOR COMPANIES WORLDWIDE
TABLE 85
STERILIZATION SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 86
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR PACKAGING & ASSEMBLY SERVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 87
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR PACKAGING & ASSEMBLY SERVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 88
PRIMARY & SECONDARY PACKAGING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 89
LABELING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 90
OTHER PACKAGING & ASSEMBLY SERVICES MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 91
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 92
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY REGION, 2023–2030 (USD MILLION)
TABLE 93
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 94
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 95
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 96
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 97
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 98
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 99
ASIA PACIFIC: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 100
ASIA PACIFIC: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 101
ASIA PACIFIC: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 102
CHINA: KEY MACROINDICATORS
TABLE 103
CHINA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 104
CHINA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 105
CHINA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 106
CHINA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 107
CHINA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 108
CHINA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 109
CHINA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 110
CHINA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 111
INDIA: KEY MACROINDICATORS
TABLE 113
INDIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 114
INDIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 115
INDIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 116
INDIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 117
INDIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 118
INDIA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 119
INDIA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 120
INDIA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 121
JAPAN: KEY MACROINDICATORS
TABLE 122
JAPAN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 123
JAPAN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 124
JAPAN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 125
JAPAN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 126
JAPAN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 127
JAPAN: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 128
JAPAN: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 129
JAPAN: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 130
MALAYSIA: KEY MACROINDICATORS
TABLE 131
MALAYSIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 132
MALAYSIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 133
MALAYSIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 134
MALAYSIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 135
MALAYSIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 136
MALAYSIA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 137
MALAYSIA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 138
MALAYSIA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 139
THAILAND: KEY MACROINDICATORS
TABLE 141
THAILAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 142
THAILAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 143
THAILAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 144
THAILAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 145
THAILAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 146
THAILAND: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 147
THAILAND: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 148
THAILAND: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 149
SOUTH KOREA: KEY MACROINDICATORS
TABLE 151
SOUTH KOREA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 152
SOUTH KOREA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 153
SOUTH KOREA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 154
SOUTH KOREA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 156
SOUTH KOREA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 157
SOUTH KOREA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 158
SOUTH KOREA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 159
SOUTH KOREA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 160
AUSTRALIA: KEY MACROINDICATORS
TABLE 161
AUSTRALIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 162
AUSTRALIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 163
AUSTRALIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 164
AUSTRALIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 165
AUSTRALIA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 166
AUSTRALIA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 167
AUSTRALIA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 168
AUSTRALIA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 169
PHILIPPINES: KEY MACROINDICATORS
TABLE 170
PHILIPPINES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 171
PHILIPPINES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 172
PHILIPPINES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 173
PHILIPPINES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 174
PHILIPPINES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 175
PHILIPPINES: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 176
PHILIPPINES: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 177
PHILIPPINES: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 178
SINGAPORE: KEY MACROINDICATORS
TABLE 179
SINGAPORE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 180
SINGAPORE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 181
SINGAPORE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 182
SINGAPORE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 183
SINGAPORE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 184
SINGAPORE: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 185
SINGAPORE: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 186
SINGAPORE: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 187
NEW ZEALAND: KEY MACROINDICATORS
TABLE 188
NEW ZEALAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 189
NEW ZEALAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 190
NEW ZEALAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 191
NEW ZEALAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 192
NEW ZEALAND: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 193
NEW ZEALAND: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 194
NEW ZEALAND: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 195
NEW ZEALAND: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 196
REST OF ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 197
REST OF ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 198
REST OF ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 199
REST OF ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 200
REST OF ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 201
REST OF ASIA PACIFIC: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 202
REST OF ASIA PACIFIC: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 203
REST OF ASIA PACIFIC: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 204
EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 205
EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 206
EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 207
EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 208
EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 209
EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 210
EUROPE: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 211
EUROPE: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 212
EUROPE: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 213
GERMANY: KEY MACROINDICATORS
TABLE 214
GERMANY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 215
GERMANY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 216
GERMANY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 217
GERMANY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 218
GERMANY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 219
GERMANY: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 220
GERMANY: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 221
GERMANY: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 222
UK: KEY MACROINDICATORS
TABLE 223
UK: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 224
UK: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 225
UK: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 226
UK: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 227
UK: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 228
UK: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 229
UK: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 230
UK: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 231
FRANCE: KEY MACROINDICATORS
TABLE 232
FRANCE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 233
FRANCE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 234
FRANCE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 235
FRANCE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 236
FRANCE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 237
FRANCE: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 238
FRANCE: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 239
FRANCE: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 240
SPAIN: KEY MACROINDICATORS
TABLE 241
SPAIN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 242
SPAIN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 243
SPAIN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 244
SPAIN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 245
SPAIN: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 246
SPAIN: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 247
SPAIN: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 248
SPAIN: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 249
ITALY: KEY MACROINDICATORS
TABLE 250
ITALY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 251
ITALY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 252
ITALY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 253
ITALY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 254
ITALY: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 255
ITALY: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 256
ITALY: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 257
ITALY: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 258
REST OF EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 259
REST OF EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 260
REST OF EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 261
REST OF EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 262
REST OF EUROPE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 263
REST OF EUROPE: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 264
REST OF EUROPE: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 265
REST OF EUROPE: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 266
NORTH AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 267
NORTH AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 268
NORTH AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 269
NORTH AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 270
NORTH AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 271
NORTH AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 272
NORTH AMERICA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 273
NORTH AMERICA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 274
NORTH AMERICA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 275
US: KEY MACROINDICATORS
TABLE 276
US: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 277
US: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 278
US: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 279
US: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 280
US: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 281
US: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 282
US: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 283
US: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 284
CANADA: KEY MACROINDICATORS
TABLE 285
CANADA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 286
CANADA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 287
CANADA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 288
CANADA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 289
CANADA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 290
CANADA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 291
CANADA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 292
CANADA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 293
LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 294
LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 295
LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 296
LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 297
LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 298
LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 299
LATIN AMERICA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 300
LATIN AMERICA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 301
LATIN AMERICA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 302
BRAZIL: KEY MACROINDICATORS
TABLE 303
BRAZIL: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 304
BRAZIL: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 305
BRAZIL: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 306
BRAZIL: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 307
BRAZIL: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 308
BRAZIL: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 309
BRAZIL: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 310
BRAZIL: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 311
MEXICO: KEY MACROINDICATORS
TABLE 312
MEXICO: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 313
MEXICO: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 314
MEXICO: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 315
MEXICO: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 316
MEXICO: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 317
MEXICO: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 318
MEXICO: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 319
MEXICO: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 320
REST OF LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 321
REST OF LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 322
REST OF LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 323
REST OF LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 324
REST OF LATIN AMERICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 325
REST OF LATIN AMERICA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 326
REST OF LATIN AMERICA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 327
REST OF LATIN AMERICA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 328
MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY REGION, 2023–2030 (USD MILLION)
TABLE 329
MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 330
MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 331
MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 332
MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 333
MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 334
MIDDLE EAST & AFRICA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 335
MIDDLE EAST & AFRICA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 336
MIDDLE EAST & AFRICA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 337
GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD MILLION)
TABLE 338
GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 339
GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 340
GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 341
GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 342
GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 343
GCC COUNTRIES: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 344
GCC COUNTRIES: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 345
GCC COUNTRIES: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 346
KSA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 347
KSA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 348
KSA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 349
KSA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 350
KSA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 351
KSA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 352
KSA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 353
KSA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 354
UAE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 355
UAE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 356
UAE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 357
UAE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 358
UAE: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 359
UAE: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 360
UAE: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 361
UAE: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 362
REST OF GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 363
REST OF GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 364
REST OF GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 365
REST OF GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 366
REST OF GCC COUNTRIES: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 367
REST OF GCC COUNTRIES: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 368
REST OF GCC COUNTRIES: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 369
REST OF GCC COUNTRIES: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 370
REST OF MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2023–2030 (USD MILLION)
TABLE 371
REST OF MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR IVD DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 372
REST OF MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET FOR DRUG DELIVERY DEVICES, BY TYPE, 2023–2030 (USD MILLION)
TABLE 373
REST OF MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE CLASS, 2023–2030 (USD MILLION)
TABLE 374
REST OF MIDDLE EAST & AFRICA: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD MILLION)
TABLE 375
REST OF MIDDLE EAST & AFRICA: DEVICE DEVELOPMENT & MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 376
REST OF MIDDLE EAST & AFRICA: QUALITY MANAGEMENT SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 377
REST OF MIDDLE EAST & AFRICA: PACKAGING & ASSEMBLY SERVICES MARKET, BY TYPE, 2023–2030 (USD MILLION)
TABLE 378
OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN MEDICAL DEVICE CONTRACT MARKET
TABLE 379
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DEGREE OF COMPETITION
TABLE 380
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: REGION FOOTPRINT
TABLE 381
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DEVICE FOOTPRINT (1/2)
TABLE 382
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DEVICE FOOTPRINT (2/2)
TABLE 383
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DEVICE CLASS FOOTPRINT
TABLE 384
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: SERVICE FOOTPRINT
TABLE 385
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DETAILED LIST OF KEY STARTUPS/SMES
TABLE 386
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
TABLE 387
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022–JUNE 2025
TABLE 388
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: DEALS, JANUARY 2022–JUNE 2025
TABLE 389
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: OTHER DEVELOPMENTS, JANUARY 2022–JUNE 2025
TABLE 390
JABIL INC.: COMPANY OVERVIEW
TABLE 391
JABIL INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 392
JABIL INC: PRODUCT APPROVALS, JANUARY 2022–JUNE 2025
TABLE 393
JABIL INC: DEALS, JANUARY 2022–JUNE 2025
TABLE 394
FLEX LTD.: COMPANY OVERVIEW
TABLE 395
FLEX LTD.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 396
FLEX LTD.: DEALS, JANUARY 2022–JUNE 2025
TABLE 397
FLEX LTD.: EXPANSIONS, JANUARY 2022–JUNE 2025
TABLE 398
PLEXUS CORP.: COMPANY OVERVIEW
TABLE 399
PLEXUS CORP.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 400
PLEXUS CORP: EXPANSIONS, JANUARY 2022–JUNE 2025
TABLE 401
SANMINA CORPORATION: COMPANY OVERVIEW
TABLE 402
SANMINA CORPORATION: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 403
SANMINA CORPORATION: DEALS, JANUARY 2022–JUNE 2025
TABLE 404
INTEGER HOLDINGS CORPORATION: COMPANY OVERVIEW
TABLE 405
INTEGER HOLDINGS CORPORATION: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 406
INTEGER HOLDINGS CORPORATION: DEALS, JANUARY 2022–JUNE 2025
TABLE 407
TE CONNECTIVITY LTD.: COMPANY OVERVIEW
TABLE 408
TE CONNECTIVITY LTD.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 409
TE CONNECTIVITY LTD: PRODUCT LAUNCHES, JANUARY 2022–JUNE 2025
TABLE 410
TE CONNECTIVITY LTD: DEALS, JANUARY 2022–JUNE 2025
TABLE 411
NIPRO CORPORATION: COMPANY OVERVIEW
TABLE 412
NIPRO CORPORATION: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 413
NIPRO CORPORATION: EXPANSIONS: JANUARY 2022–JUNE 2025
TABLE 414
CELESTICA INC.: COMPANY OVERVIEW
TABLE 415
CELESTICA INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 416
CELESTICA INC.: EXPANSIONS: JANUARY 2022–JUNE 2025
TABLE 417
CELESTICA INC.: OTHER DEVELOPMENTS, JANUARY 2022- JUNE 2025
TABLE 418
WEST PHARMACEUTICALS SERVICES, INC.: COMPANY OVERVIEW
TABLE 419
WEST PHARMACEUTICAL SERVICES, INC: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 420
WEST PHARMACEUTICAL SERVICES, INC: PRODUCT LAUNCHES, JANUARY 2022–JUNE 2025
TABLE 421
WEST PHARMACEUTICAL SERVICES, INC: DEALS, JANUARY 2022–JUNE 2025
TABLE 422
BENCHMARK ELECTRONICS INC.: COMPANY OVERVIEW
TABLE 423
BENCHMARK ELECTRONICS INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 424
BENCHMARK ELECTRONICS INC.: DEALS, JANUARY 2022–JUNE 2025
TABLE 425
BENCHMARK ELECTRONICS INC.: EXPANSIONS, JANUARY 2022–JUNE 2025
TABLE 426
RECIPHARM AB: COMPANY OVERVIEW
TABLE 427
RECIPHARM AB: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 428
RECIPHARM AB: DEALS, JANUARY 2022–JUNE 2025
TABLE 429
RECIPHARM AB: OTHER DEVELOPMENTS, JANUARY 2022–JUNE 2025
TABLE 430
GERRESHEIMER AG: COMPANY OVERVIEW
TABLE 431
GERRESHEIMER AG: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 432
GERRESHEIMER AG: DEALS, JANUARY 2022–JUNE 2025
TABLE 433
KIMBALL ELECTRONICS, INC.: COMPANY OVERVIEW
TABLE 434
KIMBALL ELECTRONICS, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 435
NORTECH SYSTEMS, INC.: COMPANY OVERVIEW
TABLE 436
NORTECH SYSTEMS, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 437
NORTECH SYSTEMS, INC.: PRODUCT APPROVALS, JANUARY 2022–JUNE 2025
TABLE 438
CARCLO PLC: COMPANY OVERVIEW
TABLE 439
CARCLO PLC: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 440
NOLATO GW, INC.: COMPANY OVERVIEW
TABLE 441
NOLATO GW, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
TABLE 442
NOLATO GW, INC.: DEALS, JANUARY 2022–JUNE 2025
TABLE 443
NEMERA: COMPANY OVERVIEW
TABLE 444
VIANT MEDICAL HOLDINGS, INC.: COMPANY OVERVIEW
TABLE 445
TECOMET, INC.: COMPANY OVERVIEW
TABLE 446
SMC LTD.: COMPANY OVERVIEW
TABLE 447
PHILLIPS-MEDISIZE CORPORATION: COMPANY OVERVIEW
TABLE 448
TESSY PLASTICS CORP.: COMPANY OVERVIEW
TABLE 449
MEHOW: COMPANY OVERVIEW
TABLE 450
TEKNI-PLEX: COMPANY OVERVIEW
TABLE 451
PETER’S TECHNOLOGY: COMPANY OVERVIEW
FIGURE 1
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: MARKETS & REGIONS COVERED
FIGURE 2
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: RESEARCH DESIGN
FIGURE 4
BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY SIDE AND DEMAND SIDE PARTICIPANTS
FIGURE 5
BREAKDOWN OF PRIMARY INTERVIEWS (SUPPLY SIDE): BY COMPANY TYPE, DESIGNATION, AND REGION
FIGURE 6
BREAKDOWN OF PRIMARY INTERVIEWS (DEMAND SIDE): BY END USER, DESIGNATION, AND REGION
FIGURE 7
SUPPLY SIDE MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS
FIGURE 8
REVENUE SHARE ANALYSIS ILLUSTRATION: FLEX, LTD.
FIGURE 9
SUPPLY SIDE ANALYSIS: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET (2024)
FIGURE 10
DEMAND-SIDE ESTIMATION FOR MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
FIGURE 11
CAGR PROJECTIONS FROM ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES (2025–2030)
FIGURE 12
CAGR PROJECTIONS: SUPPLY SIDE ANALYSIS
FIGURE 13
TOP-DOWN APPROACH
FIGURE 14
MARKET DATA TRIANGULATION METHODOLOGY
FIGURE 15
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY DEVICE, 2025 VS. 2030 (USD MILLION)
FIGURE 16
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY CLASS OF DEVICE, 2025 VS. 2030 (USD MILLION)
FIGURE 17
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, BY SERVICE, 2025 VS. 2030 (USD MILLION)
FIGURE 18
GEOGRAPHICAL SNAPSHOT OF MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
FIGURE 19
TECHNOLOGICAL ADVANCEMENTS IN MEDICAL DEVICE MODALITIES TO DRIVE MARKET
FIGURE 20
CHINA AND CLASS I SEGMENT ACCOUNTED FOR LARGEST SHARE OF ASIA PACIFIC MARKET IN 2024
FIGURE 21
EMERGING ECONOMIES SHOW HIGHEST GROWTH DURING FORECAST PERIOD
FIGURE 22
ASIA PACIFIC COUNTRIES TO EMERGE AS GROWTH HOTSPOTS
FIGURE 23
ASIA PACIFIC TO DOMINATE MARKET IN 2030
FIGURE 24
MARKET DYNAMICS: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET
FIGURE 25
GDP GROWTH FORECAST: COMPARISON OF INDIA, CHINA, US, AND GERMANY, 2022 VS. 2030 (FORECAST)
FIGURE 26
FACTORS DRIVING DEMAND FOR CONTRACT MANUFACTURING
FIGURE 27
HEALTHCARE EXPENDITURE (%), BY COUNTRY (2010 TO 2022)
FIGURE 28
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: PORTER’S FIVE FORCES ANALYSIS
FIGURE 29
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR MEDICAL DEVICES
FIGURE 30
KEY BUYING CRITERIA FOR MEDICAL DEVICES
FIGURE 31
VALUE CHAIN ANALYSIS: MEDICAL DEVICE CONTRACT MANUFACTURING (2024)
FIGURE 32
PATENT PUBLICATION TRENDS, JURISDICTION AND TOP APPLICANT ANALYSIS (JANUARY 2015–JUNE 2025)
FIGURE 33
TOP APPLICANT COUNTRIES/REGIONS FOR MEDICAL DEVICE PATENTS (JANUARY 2015–JUNE 2025)
FIGURE 34
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: SUPPLY CHAIN (2024)
FIGURE 35
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET ECOSYSTEM, 2024
FIGURE 36
ADJACENT MARKETS FOR MEDICAL DEVICE CONTRACT MANUFACTURING
FIGURE 37
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: INVESTMENT/VENTURE CAPITAL SCENARIO, 2020–2024
FIGURE 38
US: NUMBER OF CASES OF SEXUALLY TRANSMITTED DISEASES, 2023 V/S 2024
FIGURE 39
STAGES INVOLVED IN CONTRACT MANUFACTURING OF MEDICAL DEVICES
FIGURE 40
DEVICE PROCESS DEVELOPMENT SERVICES
FIGURE 41
FINAL ASSEMBLY SERVICES IN GLOBAL VALUE CHAIN OF MEDICAL DEVICES
FIGURE 42
MDCM MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
FIGURE 43
ASIA PACIFIC: MEDICAL DEVICE CONTRACT MANUFACTURING MARKET SNAPSHOT
FIGURE 44
REVENUE ANALYSIS OF KEY PLAYERS IN MEDICAL DEVICE CONTRACT MANUFACTURING MARKET (2020−2024)
FIGURE 45
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET SHARE, BY KEY PLAYER, (2024)
FIGURE 46
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
FIGURE 47
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: COMPANY FOOTPRINT
FIGURE 48
MEDICAL DEVICE CONTRACT MANUFACTURING MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
FIGURE 49
EV/EBITDA OF KEY VENDORS
FIGURE 50
YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
FIGURE 51
BRAND/PRODUCT COMPARATIVE ANALYSIS
FIGURE 52
R&D ASSESSMENT OF KEY PLAYERS IN MEDICAL DEVICE CONTRACT MANUFACTURING MARKET, 2022–2024
FIGURE 53
JABIL INC.: COMPANY SNAPSHOT (2024)
FIGURE 54
FLEX LTD.: COMPANY SNAPSHOT (2024)
FIGURE 55
PLEXUS CORP.: COMPANY SNAPSHOT (2024)
FIGURE 56
SANMINA CORPORATION: COMPANY SNAPSHOT (2024)
FIGURE 57
INTEGER HOLDINGS CORPORATION: COMPANY SNAPSHOT (2024)
FIGURE 58
TE CONNECTIVITY LTD.: COMPANY SNAPSHOT (2024)
FIGURE 59
NIPRO CORPORATION: COMPANY SNAPSHOT (2024)
FIGURE 60
CELESTICA INC.: COMPANY SNAPSHOT (2024)
FIGURE 61
WEST PHARMACEUTICAL SERVICES, INC.: COMPANY SNAPSHOT (2024)
FIGURE 62
BENCHMARK ELECTRONICS INC.: COMPANY SNAPSHOT (2024)
FIGURE 63
RECIPHARM AB: COMPANY SNAPSHOT (2024)
FIGURE 64
GERRESHEIMER AG: COMPANY SNAPSHOT (2024)
FIGURE 65
KIMBALL ELECTRONICS, INC.: COMPANY SNAPSHOT (2024)
FIGURE 66
NORTECH SYSTEMS, INC.: COMPANY SNAPSHOT (2024)
FIGURE 67
CARCLO PLC: COMPANY SNAPSHOT (2024)
FIGURE 68
NOLATO GW, INC.: COMPANY SNAPSHOT (2024)

































Christine
Mar, 2022
How the emerging trends in the Medical Device Contract Manufacturing Market are benefiting the global Medical Deices industry?.
Rachel
Mar, 2022
I need detailed information on the geographical segmentation of the Global Medical Device Contract Manufacturing Market.
Jamie
Mar, 2022
Can you please elaborate on the recent developments in the Global Medical Device Contract Manufacturing Market?.