This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, fundamental market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, investor presentations, SEC filings of companies and publications from government sources [such as National Institutes of Health (NIH), US FDA, US Census Bureau, World Health Organization (WHO), International Trade Administration (ITA), Global Burden of Disease Study, and Centers for Medicare and Medicaid Services (CMS) were referred to identify and collect information for the global biopsy device market study. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in the biopsy device market. The primary sources from the demand side included industry experts, purchase & sales managers, doctors, and personnel from research organizations. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends and key market dynamics.
A breakdown of the primary respondents is provided below:
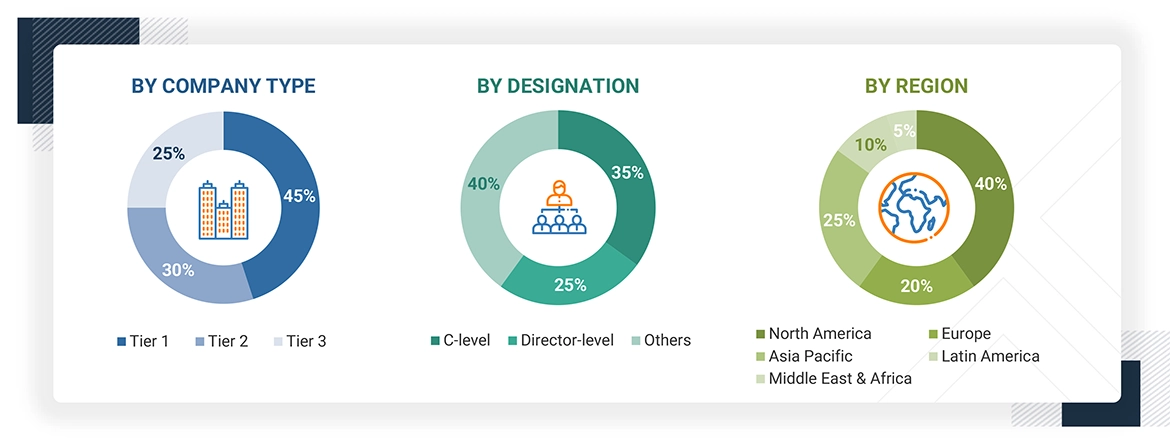
*C-level primaries include CEOs, CFOs, COOs, and VPs.
*Others include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
Note: Companies are classified into tiers based on their total revenue. As of 2023, Tier 1 = >USD 10.00 billion, Tier 2 = USD 1.00 billion to USD 10.00 billion, and Tier 3 = < USD 1.00 billion.
Source: MarketsandMarkets Analysis
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
For the global market value, annual revenues were calculated based on the revenue mapping of major product manufacturers and OEMs active in the global biopsy device equipment market. All the major product manufacturers were identified at the global and/or country/regional level. Revenue mapping for the respective business segments/sub-segments was done for the major players. Also, the global biopsy device market was split into various segments and sub-segments based on:
-
List of major players operating in the products market at the regional and/or country level
-
Product mapping of various vaccine storage equipment manufacturers at the regional and/or country level
-
Mapping of annual revenue generated by listed major players from biopsy device (or the nearest reported business unit/product category)
-
Extrapolation of the revenue mapping of the listed major players to derive the global market value of the respective segments/subsegments
-
Summation of the market value of all segments/subsegments to arrive at the global biopsy device market
The above-mentioned data was consolidated and added with detailed inputs and analysis from MarketsandMarkets and presented in this report.
Market Size Estimation (Bottom Up approach & Top down approach)
Data Triangulation
After arriving at the overall size of the global biopsy device market through the above-mentioned methodology, this market was split into several segments and subsegments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact market value data for the key segments and subsegments. The extrapolated market data was triangulated by studying various macro indicators and regional trends from both demand- and supply-side participants.
Market Definition
Biopsy devices are medical instruments used to extract tissue samples from the body for diagnostic examination, primarily to detect cancer, infections, or other diseases. These devices include biopsy needles, forceps, punches, and vacuum-assisted biopsy systems, commonly used in procedures such as needle biopsy, surgical biopsy, and endoscopic biopsy.
Stakeholders
-
Manufacturers of biopsy devices
-
Biopsy device distributors
-
Healthcare service providers
-
Various research associations related to biopsy devices
-
Research institutes
-
Venture capitalists and investors
-
Various research and consulting companies
-
World Health Organization (WHO)
-
Organization for Economic Co-operation and Development (OECD)
-
National Institutes of Health (NIH)
-
Centers for Disease Control and Prevention (CDC)
-
Annual Reports/SEC Filings, Investor Presentations, and Press Releases of Key Players
-
White Papers, Journals/Magazines, and News Articles
-
Paid Databases, such as Factiva, D&B Hoovers, and Bloomberg Business
Report Objectives
-
To describe, analyze, and forecast the biopsy device market by product, by technology, by application, by end user, and region
-
To describe and forecast the biopsy device market in key regions—North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa.
-
To provide detailed information regarding the drivers, restraints, opportunities, and challenges influencing the market growth
-
To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall market
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for market players
-
To profile key players and comprehensively analyze their market shares and core competencies in the biopsy device market
-
To analyze competitive developments such as partnerships, collaborations, agreements & acquisitions, product launches, expansions, and R&D activities in the biopsy device market
-
To analyze the impact of AI/Gen AI on the biopsy device market in terms of its capabilities, potential, use cases, and future



Growth opportunities and latent adjacency in Biopsy Devices Market