This study involved three major activities in estimating the size of the smart pills market. Exhaustive secondary research was done to collect information on the market, peer market, and parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across value chains through primary research. The bottom-up approach was employed to estimate the overall market size. After that, market breakdown and data triangulation were used to estimate the market size of segments and sub-segments.
Secondary Research
The secondary research process involved the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the clinical decision support system market. It was also used to obtain important information about the key players and market classification and segmentation according to industry trends to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations operating in the smart pills market. The primary sources from the demand side included industry experts, consultants, healthcare providers, hospital administration, and government bodies. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends and key market dynamics.
Breakdown of Primary Participants:
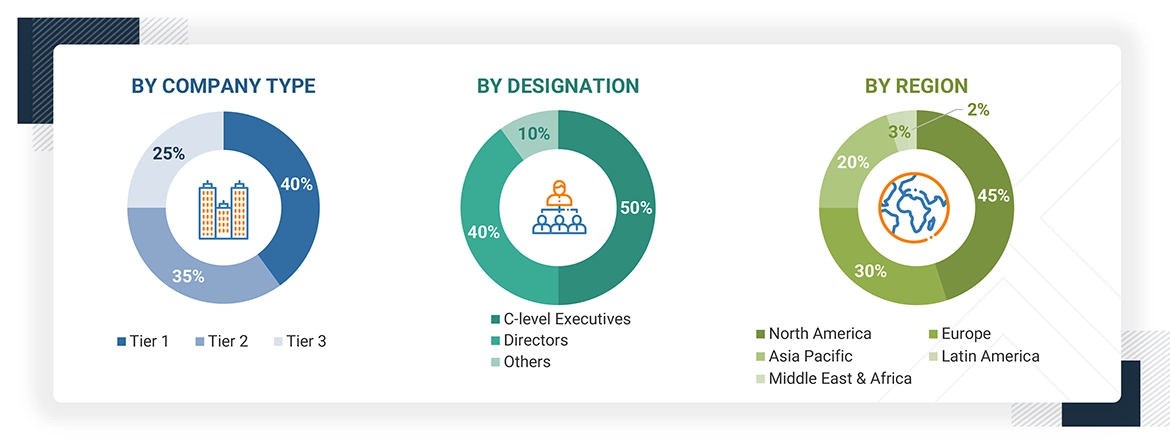
Note 1: Others include sales managers, marketing managers, and product managers.
Note 2: C-level executives include CEOs, COOs, CTOs, and VPs.
Note 3: Tiers of companies are defined on the basis of their total revenues in 2023. Tier 1 = >USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, and Tier 3 = < USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the smart pills market. These methods were also used extensively to estimate the size of various subsegments in the market.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes as explained above—the market was split into several segments and sub-segments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, the data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the smart pills market.
Market Definition
"Smart pills" refer to miniature electronic devices that are shaped and designed like pharmaceutical capsules, but are equipped with advanced functions such as sensing, imaging, and drug delivery. These devices may incorporate biosensors, image sensors, pH sensors, or chemical sensors. When ingested, they traverse the gastrointestinal tract to gather valuable information that is otherwise challenging to obtain. Unlike implantable or wearable sensors, these ingestible sensors can be easily eliminated from the body after use.
Stakeholders
-
Smart pills manufacturers
-
Healthcare providers (physician practices, diagnostic centers, and outpatient clinics)
-
Government bodies
-
Software Developers and Data Analysts
-
Corporate entities
-
Pharmaceutical companies
-
Hospitals
-
Research and consulting firms
-
Venture Capitalists
Report Objectives
-
To define, describe, and forecast the smart pills market based on the application, target area, disease indication, end user, and region.
-
To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
-
To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall smart pills market
-
To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders.
-
To forecast the size of the market segments with respect to five main regions, namely, North America, Europe, the Asia Pacific, Latin America, and Middle East & Africa.
-
To profile the key players and analyze their market shares and core competencies
-
To track and analyze competitive developments such as product launches & enhancements, agreements, collaborations, partnerships, and expansions in the smart pills market.
-
To benchmark players within the market using the proprietary "Company Evaluation Quadrant " framework, which analyzes market players on various parameters within the broad categories of business and product strategy.



Growth opportunities and latent adjacency in Smart Pills Market