The study involved four major activities to estimate the current size of the apheresis market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. Thereafter, market breakdown and data triangulation procedures were used to estimate the market size of the segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to in order to identify and collect information for the study of apheresis Market. It was also used to obtain important information about the top players, market classification, and segmentation according to industry trends to the bottom-most level, geographic markets, and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
Extensive primary research was conducted after obtaining information regarding the apheresis market scenario through secondary research. Several primary interviews were conducted with market experts from both the demand and supply sides across major countries of North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. Primary data was collected through questionnaires, emails, and telephonic interviews. The primary sources from the supply side included various industry experts, such as Chief X Officers (CXOs), Vice Presidents (VPs), Directors from business development, marketing, product development/innovation teams, and related key executives from manufacturers; distributors operating in the apheresis market.; and key opinion leaders.
Primary interviews were conducted to gather insights such as market statistics, data on revenue collected from the products and services, market breakdowns, market size estimations, market forecasting, and data triangulation. Primary research also helped in understanding the various trends related to technology, application, vertical, and region. Stakeholders from the demand side customers/end users who are using infection control products were interviewed to understand the buyer’s perspective on the suppliers, products, and their current usage and the future outlook of their business, which will affect the overall market.
The following is a breakdown of the primary respondents:
Breakdown of Primary Participants:
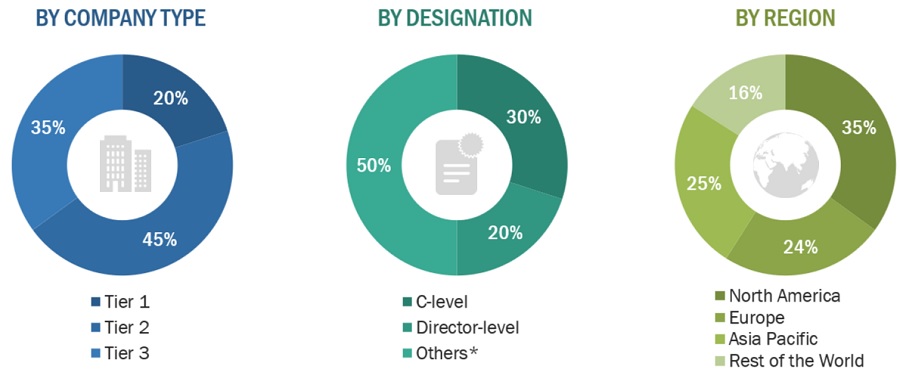
Note 1: C-level primaries include CEOs, COOs, CTOs, and VPs.
Note 2: Other primaries include sales managers, marketing managers, and product managers.
Note 3: Companies are classified into tiers based on their total revenue.
As of 2023: Tier 1=>USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, Tier 3=<USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The research methodology used to estimate the size of the apheresis market includes the following details.
The market sizing of the market was undertaken from the global side.
Country-level Analysis: The size of the apheresis market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products in the overall apheresis market was obtained from secondary data and validated by primary participants to arrive at the total apheresis market. Primary participants further validated the numbers.
Geographic market assessment (by region & country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by end users and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated through industry experts contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall apheresis market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Global Apheresis Market Size: Top-Down Approach

To know about the assumptions considered for the study, Request for Free Sample Report
Global Apheresis Market Size: Bottom-Up Approach

Data Triangulation
After arriving at the overall market size—using the market size estimation processes—the market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and sub-segment, the data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the apheresis industry.
Market Definition:
The apheresis market encompasses the global business sphere surrounding the development, production, distribution, and use of apheresis equipment and related consumables. This specialized technology enables the separation and collection of specific blood components, such as plasma, platelets, or white blood cells, from a whole blood donation. These collected components are then used in various therapeutic applications, including treating blood disorders, autoimmune diseases, and neurological conditions.
Key Stakeholders
-
Apheresis devices manufacturers and distributors
-
Hospitals and transfusion centers
-
Therapeutic apheresis service providers
-
Blood banks and blood collection centers
-
Plasma fractionation companies
-
Healthcare providers
-
Academic research institutes
-
Pharmaceutical companies
-
Contract research organizations (CROs)
-
Market research & consulting firms
Report Objectives
-
To define, describe, segment, and forecast the global apheresis market by product, technology, procedure, application, end user, and region.
-
To provide detailed information about the factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
-
To analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall apheresis market
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players.
-
To forecast the size of the apheresis market in five main regions along with their respective key countries, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
-
To profile key players in the global apheresis market and comprehensively analyze their core competencies2 and market shares.
-
To track and analyze competitive developments such as acquisitions, expansions, partnerships, agreements, and collaborations; and product launches and approvals.
-
To benchmark players within the apheresis market using the "Competitive Leadership Mapping" framework, which analyzes market players on various parameters within the broad categories of business and product strategy.
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
-
Product Analysis: Product matrix, which gives a detailed comparison of the product portfolios of each company
-
Geographic Analysis: Further breakdown of the Latin American apheresis into specific countries and, the Middle East, and Africa apheresis into specific countries and further breakdown of the European apheresis into specific countries



Growth opportunities and latent adjacency in Apheresis Market