To determine the current size of the IVD reagents market, this study engaged in four main activities. A comprehensive study was conducted using secondary research methods to gather data about the market, its parent market, and its peer markets. The next stage involved conducting primary research to confirm these conclusions, assumptions, and sizing with industry experts throughout the value chain. A combination of top-down and bottom-up methods was used to assess the overall market size. The market sizes of segments and subsegments were then estimated using data triangulation techniques and market breakdown.
The four steps involved in estimating the market size are
Secondary Research
Within the secondary data collection process, a range of secondary sources were reviewed so as to identify and gather data for this study, including regulatory bodies, databases (World Bank, World Health Organization, Centers for Disease Control and Prevention, and the US Food and Drug Administration); corporate filings (annual reports, SEC filings, investor presentations, and financial statements); research journals; press releases; and trade, business, and professional associations.
Primary Research
A thorough method was used in the primary research phase, which involved interviewing a wide range of sources from the supply and demand sides. The purpose of these interviews was to obtain the quantitative and qualitative information needed to put up this report. The main sources of information were industry experts from both core and related sectors, as well as preferred service provider, pioneers in technology, and organizations involved in every aspect of this industry's value chain. A variety of primary respondents, including important industry stakeholders, subject-matter experts, C-level executives from crucial market companies, and industry advisers, were carefully questioned in-depth. The goal was to gather and validate important qualitative and quantitative insights and to conduct a thorough assessment of future possibilities.
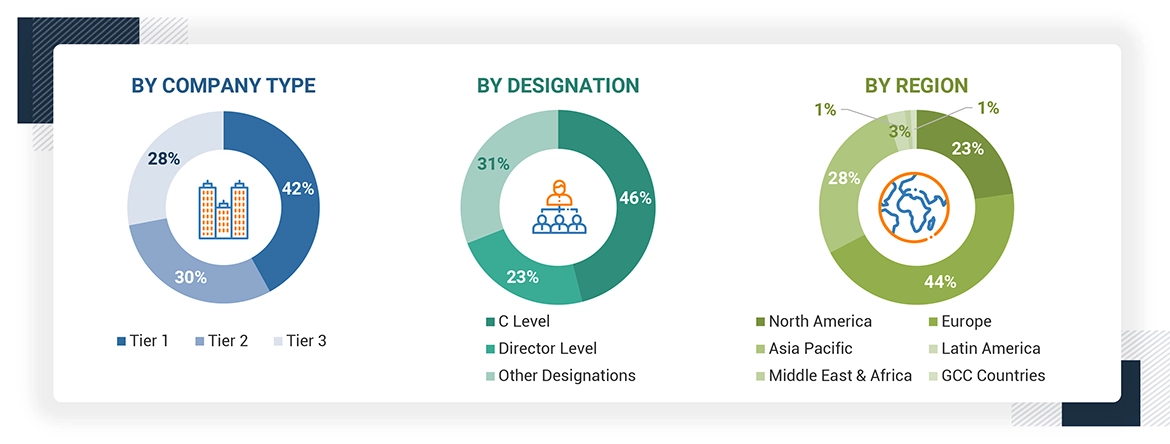
Note 1: Other designations include sales, marketing, and product managers.
Note 2: Tiers are defined based on a company’s total revenue. As of 2024, Tier 1= >USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, and Tier 3 = < USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
|
COMPANY NAME |
DESIGNATION |
|
Enzene Biosciences Ltd. |
Scientist |
|
Danaher Corporation |
Director of Research & Development |
|
Thermo Fisher Scientific Inc. |
Sales Manager |
Market Size Estimation
All major product providers offering various products and services were identified at the global/regional level. Revenue mapping was done for the major players and was extrapolated to arrive at the global market value of each type of segment. The market value of the IVD reagents market was also split into various segments and subsegments at the region and country level based on:
-
Offering mapping of various service provider for each type in the IVD reagents market at the regional and country level
-
Relative adoption pattern of each IVD reagents market among key application segments at the regional and/or country-level
-
Detailed primary research to gather qualitative and quantitative information related to segments and subsegments at the regional and/or country level.
-
Detailed secondary research to gauge the prevailing market trends at the regional and/or country level
Data Triangulation
After arriving at the overall market size applying the process mentioned above, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
In vitro diagnostic (IVD) reagents are chemicals or substances used to detect, measure, or produce a reaction with components of a sample in an IVD test. They are essential for accurately detecting biological markers or analytes in samples.
Stakeholders
-
IVD Reagents Manufacturers
-
Pathologists and Pathology Laboratories
-
Distributors of IVD Products
-
Hospitals and Clinics
-
Healthcare Institutions
-
Research Institutes
-
Market Research and Consulting Firms
-
Venture Capitalists and Investors
Report Objectives
-
To define, describe, segment, and forecast the global IVD reagents market by type, technology, application, test type, end user, and region
-
To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
-
To analyze the micro markets with respect to individual growth trends, prospects, and contributions to the overall IVD reagents market
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
-
To forecast the size of the market segments with respect to six regions: North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa, and the GCC Countries
-
To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
-
To benchmark players within the market using the proprietary Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business and product excellence



Growth opportunities and latent adjacency in IVD Reagents Market