This study involved four major activities in estimating the current size of the companion diagnostics market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate segments and subsegments' market size.
The objective of the study is to analyze the key market dynamics, such as drivers, opportunities, challenges, restraints, and key player strategies. To track company developments such as acquisitions, product launches, expansions, collaborations, agreements, and partnerships of the leading players, the competitive landscape of the companion diagnostics market to analyze market players on various parameters within the broad categories of business and product strategy. Top-down and bottom-up approaches were used to estimate the market size. To estimate the market size of segments and subsegments, market breakdown and data triangulation were used.
The four steps involved in estimating the market size are:
Collecting Secondary Data
The secondary research data collection process involves the usage of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B), annual reports, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the companion diagnostics market. A database of the key industry leaders was also prepared using secondary research.
Collecting Primary Data
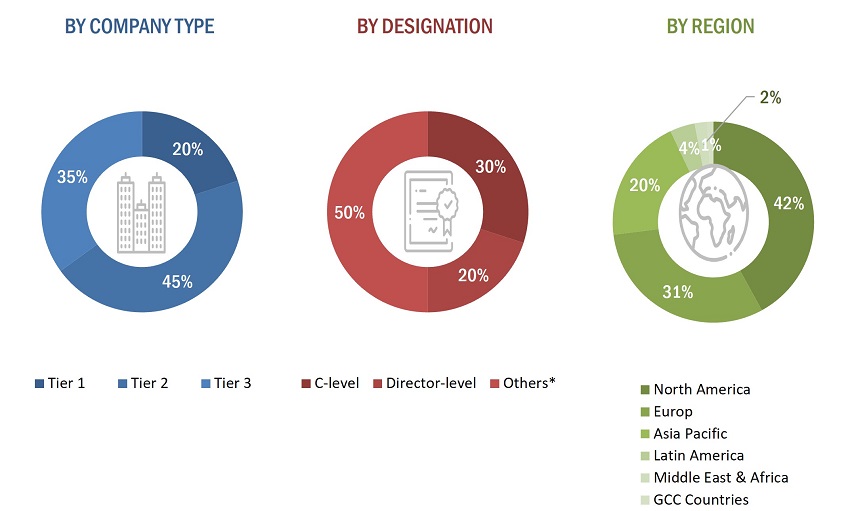
The primary research data was conducted after acquiring knowledge about the companion diagnostics market scenario through secondary research. A significant number of primary interviews were conducted with stakeholders from both the demand side (such as hospitals, clinics and ambulatory surgery centers (ASCs)) and supply side (such as included various industry experts, such as Directors, Chief X Officers (CXOs), Vice Presidents (VPs) from business development, marketing and product development teams, product manufacturers, wholesalers, channel partners, and distributors) across major countries of North America, Europe, Asia Pacific, the Middle East & Africa, Latin America and the GCC Countries. Approximately 40% of the primary interviews were conducted with stakeholders from the demand side, while those from the supply side accounted for the remaining 60%. Primary data for this report was collected through questionnaires, emails, and telephonic interviews.
A breakdown of the primary respondents is provided below:
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
All major product manufacturers offering various companion diagnostics products were identified at the global/regional level. Revenue mapping was done for the major players and was extrapolated to arrive at the global market value of each type of segment. The market value companion diagnostics market was also split into various segments and subsegments at the region and country level based on:
-
Product mapping of various manufacturers for each type of companion diagnostics market at the regional and country-level.
-
Relative adoption pattern of each companion diagnostics market among key application segments at the regional and/or country-level.
-
Detailed primary research to gather qualitative and quantitative information related to segments and subsegments at the regional and/or country-level.
-
Detailed secondary research to gauge the prevailing market trends at the regional and/or country-level
Global Companion Diagnostics Market Size: Top-Down Approach

To know about the assumptions considered for the study, Request for Free Sample Report
Data Triangulation
After arriving at the overall market size applying the process mentioned above, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the companion diagnostics device industry.
Market Definition
A companion diagnostic (CDx) assay is an in vitro diagnostic device (IVD) that is used to make biomarker assessments to identify whether a patient with certain chronic diseases, such as cancer, infectious diseases, and cardiovascular diseases, could benefit from a particular drug. CDx uses technologies such as polymerase chain reaction, next-generation sequencing, immunohistochemistry, and in situ hybridization. The biomarker assessment enable physicians to identify a specific biomarker that could indicate the patients’ most likely outcome-based combination of therapies or even a specific treatment regimen.
Key Stakeholders
-
Manufacturing Companies of Companion diagnostics and Related Devices
-
Original Equipment Manufacturers
-
Suppliers and Distributors of Electroceutical Devices
-
Healthcare Service Providers
-
Teaching Hospitals and Academic Medical Centers (AMCs)
-
Health Insurance Payers
-
Research and Consulting Firms
-
Medical Research Institutes
-
Healthcare Institutes/Providers (Hospitals, Medical Groups, Physicians’ Practices, Diagnostic Centers, and Outpatient Clinics)
-
Venture Capitalists
-
Community Centers
Report Objectives
-
To define, describe, segment, and forecast the companion diagnostics market by product & service, technology, indication, sample type, end user, and region.
-
To provide detailed information regarding the major factors influencing the market growth (drivers, restraints, opportunities, and challenges).
-
To analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall market.
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players.
-
To forecast the size of the market segments with respect to six regions, namely, North America, Europe, the Asia Pacific, Middle East & Africa, Latin America, and GCC countries.
-
To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies.
-
To track and analyze company developments such as acquisitions, collaborations, partnerships, agreements, and product launches & approvals in the companion diagnostics market
-
To benchmark players within the companion diagnostics market using the Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business strategy, market share, and product offerings
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Country Information
-
Companion diagnostics market size and growth rate estimates for countries in the Rest of Europe, the Rest of Asia Pacific, the Rest of Latin America, and Middle East & Africa.
Company Profiles
-
Product portfolio matrix for leading market players.



Alisa
Jul, 2022
What is the Companion Diagnostics Market trend by Product (Assays, Kits, Software & Services), Technology (PCR, NGS, ISH, IHC), Indication (Breast, Blood, Lung, Colorectal Cancer, Neurology Diseases), End User (Pharma Companies, CROs), Regional Analysis - Global Forecast to 2031.
Alan
Dec, 2022
Companion Diagnostics Market Report provides the global forecasts for the next five years. Companies like ResearchDx is profiled as one of the key players in this report. Included the comprehensive 360 degree competitive mapping by profiling major players of Companion Diagnostics Market. Key Questions Addressed in the Report: • Where will all these products & services take the industry in the mid to long term? • What are the growth opportunities in the Companion Diagnostics Market across major regions in the future? • Which are the key players in the market and how intense is the competition? • What are the current industry developments for companion diagnostics? We have given a comprehensive 360 degree competitive mapping by profiling major players of Companion Diagnostics Market..