The study involved four major activities to estimate the current size of the cardaic tissue engineering market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. Thereafter, market breakdown and data triangulation procedures were used to estimate the market size of the segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for the study of Cardiac Tissue Engineering Market. It was also used to obtain important information about the top players, market classification, and segmentation according to industry trends to the bottom-most level, geographic markets, and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
Extensive primary research was conducted after obtaining information regarding the cardiac tissue engineering market scenario through secondary research. Several primary interviews were conducted with market experts from both the demand and supply sides across major countries of North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. Primary data was collected through questionnaires, emails, and telephonic interviews. The primary sources from the supply side included various industry experts, such as Chief X Officers (CXOs), Vice Presidents (VPs), Directors from business development, marketing, product development/innovation teams, and related key executives from manufacturers; distributors operating in the Cardiac Tissue Engineering market.; and key opinion leaders.
Primary interviews were conducted to gather insights such as market statistics, data on revenue collected from the products and services, market breakdowns, market size estimations, market forecasting, and data triangulation. Primary research also helped in understanding the various trends related to technology, application, vertical, and region. Stakeholders from the demand side customers/end users who are using infection control products were interviewed to understand the buyer’s perspective on the suppliers, products, and their current usage and the future outlook of their business, which will affect the overall market.
The following is a breakdown of the primary respondents:
Breakdown of Primary Participants:
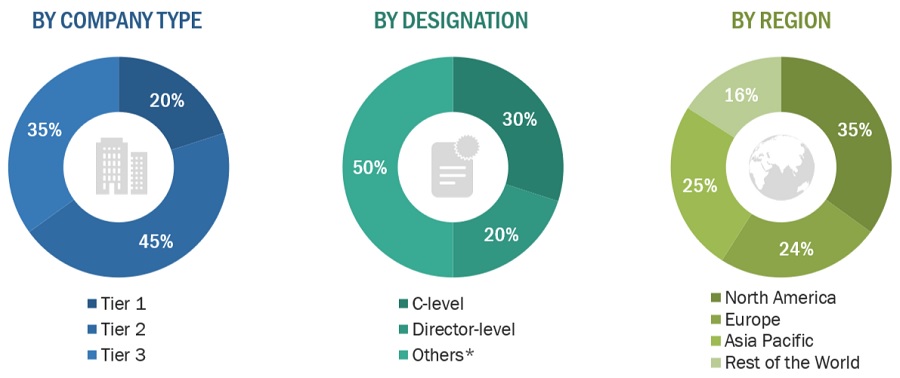
Note 1: C-level primaries include CEOs, COOs, CTOs, and VPs.
Note 2: Other primaries include sales managers, marketing managers, and product managers.
Note 3: Companies are classified into tiers based on their total revenue.
As of 2023: Tier 1=>USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, Tier 3=<USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The research methodology used to estimate the size of the cardiac tissue engineering market includes the following details.
The market sizing of the market was undertaken from the global side.
Country-level Analysis: The size of the Cardiac Tissue Engineering market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products in the overall cardiac tissue engineering market was obtained from secondary data and validated by primary participants to arrive at the total cardiac tissue engineering market. Primary participants further validated the numbers.
Geographic market assessment (by region & country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by end users and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated through industry experts contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall Cardiac Tissue Engineering market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Global Cardiac Tissue Engineering Market Size: Top-Down Approach

To know about the assumptions considered for the study, Request for Free Sample Report
Global Cardiac Tissue Engineering Market Size: Bottom-Up Approach

Data Triangulation
After arriving at the overall market size—using the market size estimation processes—the market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and sub-segment, the data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the Cardiac Tissue Engineering industry.
Market Definition:
Cardiac tissue engineering is an interdisciplinary field focused on developing functional heart tissues to repair or regenerate damaged cardiac tissue. It combines biological, engineering, and medical principles to create scaffolds seeded with stem cells or primary cardiac cells, forming tissues that mimic the heart's structure and function. Key elements include designing biocompatible scaffolds, utilizing bioreactors for tissue culture, ensuring vascularization, and achieving integration with the host's heart tissue. This innovative approach aims to overcome the limitations of current heart disease treatments, offering potential for improved heart function and new therapeutic options for patients with cardiac conditions.
Key Stakeholders
-
Healthcare service providers (including hospitals, clinics)
-
Pharmaceutical and biotechnological centers
-
Research institutes and universities
Report Objectives
-
To define, describe, segment, and forecast the global Cardiac Tissue Engineering market by material type, product type, application, end-user, and region.
-
To strategically analyze the industry trends, technology trends, pricing analysis, regulatory scenario, supply/value chain, ecosystem mapping, Porter’s Five Forces, patent analysis, key stakeholders and buying criteria, and conferences and events
-
To provide detailed information regarding the major factors influencing the growth of this market (such as drivers, restraints, opportunities, and challenges)
-
To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall cardiac Tissue engineering market
-
To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders.
-
To forecast the market size based on six key regions: North America, Europe, Asia Pacific, Latin America, and Middle East & Africa
-
To strategically profile key players in the cardiac Tissue engineering market and comprehensively analyze their core competencies.
-
To track and analyze competitive developments such as product/service launches & approvals, partnerships, agreements, collaborations, joint ventures, expansions, and acquisitions in the overall cardiac Tissue engineering market.
-
To benchmark players within the market using the proprietary “Competitive Leadership Mapping” framework, which analyzes market players on various parameters within the broad categories of business and product/service strategy.
-
To analyze the impact of the recession on the cardiac Tissue engineering market.
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
-
Product Analysis: Product matrix, which gives a detailed comparison of the product portfolios of each company
-
Geographic Analysis: Further breakdown of the Latin American Cardiac Tissue Engineering into specific countries and, the Middle East, and Africa Cardiac Tissue Engineering into specific countries and further breakdown of the European Cardiac Tissue Engineering into specific countries,



Growth opportunities and latent adjacency in Cardiac Tissue Engineering Market