Rotator Cuff Injury Treatment Market by Modality (Surgical (Arthroscopy, Shoulder Replacement, Tendon Repair), Physiotherapy (Braces, Cold Compression), Drug Therapeutics (Anti-inflammatory Drugs, Injections), Orthobiologics) - Global Forecast to 2026
Market Growth Outlook Summary
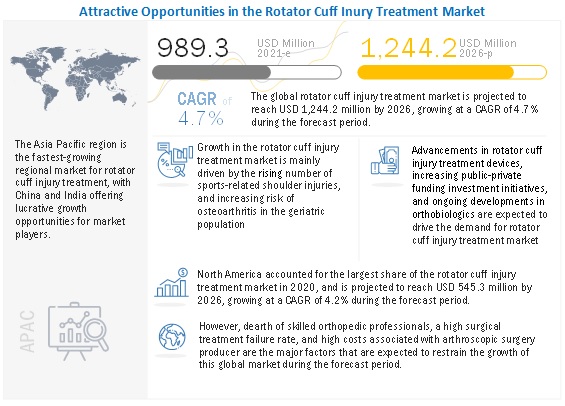
The global rotator cuff injury treatment market growth forecasted to transform from $989.3 million in 2021 to $1,244.2 million by 2026, driven by a CAGR of 4.7%. The key factors fueling the growth of this market include an increase in sports-related shoulder injuries, technological advancements in arthroscopic surgical devices, increase in aging population with osteoarthritis, strong focus of players towards launch of highly advanced arthroscopic treatment devices. On the other hand, a dearth of skilled professionals, a high surgical treatment failure rate, and its high cost are major challenges faced by market players providing rotator cuff injury treatment products.
To know about the assumptions considered for the study, Request for Free Sample Report
Rotator Cuff Injury Treatment Market Dynamics
Driver: Rising number of sports-related shoulder injuries
Sports and shoulder-related physical activities can cause high stress on the shoulder leading to the development of rotator cuff tendonitis, resulting in shoulder pain and weakness, further limiting the movement of the arm. The upper extremities constitute one-third of the proportion of sports injuries. Athletes engaged in sports like baseball, tennis, handball, and volleyball experience an acute episode of trauma or such as falling. Such episodes can increase the severity of shoulder injuries, ranging from rotator cuff contusions and tendinopathies development in rotator cuff tears. Around 3.5 million sports injuries are registered in the US annually (Source: National Electronic Injury Surveillance System, data as of 2019).
Restraint: High costs associated with arthroscopic surgical procedures
The high cost of arthroscopic surgery is a major concern, especially in emerging markets like China and India, where only a smaller population is under insurance coverage. The average cost of outpatient shoulder arthroscopy treatment in India can range between USD 3000 to USD 5000. Such costly treatments have reduced the affordability of shoulder arthroscopy are limiting the growth of this market. Moreover, surgical settings across low resource income nations are highly reluctant to adopt novel products owing to a lack of awareness and affordability, thus expecting to hinder the growth of this market to a certain extent.
Opportunity: Novel orthobiologics in pipeline
Regenerative biologics is a rapidly growing segment that is anticipated to change the facet of sports orthopedics significantly. Despite the development of advanced sutures, the high failure rates encourage many researchers to opt for biological therapy to stimulate the healing of an injury. Many players such as Nephew, Zimmer, Johnson & Johnson (through its DePuy Division), and Stryker are strongly shifting their focus on expanding their product offering in this domain. Moreover, the emergence of many new players in the development of innovative regenerative biologics is likely to result in potentially promising treatment options for rotator cuff injuries.
For instance: In March 2020, Ortho Regenerative Technologies Inc. announced the positive results from a pivotal preclinical study of ORTHO-R Biopolymer. This mucoadhesive CHITOSAN-based biopolymer matrix acts as a biodegradable scaffold. It is mixed with the patients PRP to develop biologics which further aids in increasing the healing rates of tendon relator injuries. With continued advanced development in orthobiologics, invasive orthopedic surgeries are expected to shift towards a minimally invasive treatment approach that offers high tendon healing rates
Challenge: High failure rates of rotator cuff surgical treatment
The incidence of rotator cuff injuries is increasing and requires immediate surgical intervention to repair the tear. However, there is still a growing failure rate in the surgical treatment of rotator cuffs, thus being a major concern. Despite improvements in rotator cuff surgery techniques, the chances of re-tear rate remain above 20%, increasing with the severity of the tear, hindering the overall growth of the global market.
The surgical or curative treatment to capture the largest share in rotator cuff injury treatment industry, by type during the forecast period.
The surgical treatment segment is expected to hold the largest share of the rotator cuff injury treatment market, during the forecast period. This is attributable to the high adoption due to the increasing prevalence of rotator cuff injuries majorly among sportspersons and the availability of medical reimbursements for surgical treatment for rotator cuff rupture in the developed nations.
Additionally, the increase in the preference of shoulder arthroscopy for the treatment rotator cuff tear among surgeons and the growing awareness about the benefits associated with an arthroscopic repair such as lower risk of complications, quick recovery, low rate of post surgical infections are likely to play a significant role in propelling the growth of the segment.
To know about the assumptions considered for the study, download the pdf brochure
North America is the largest regional market for rotator cuff injury treatment industry
North America capture the largest share of the rotator cuff injury treatment market. This is attributed to the continuous development and commercialization of novel rotator cuff injury treatment products, favorable reimbursement and insurance coverage for rotator cuff repair treatment, supportive government regulations for product commercialization, and significant participation in sports.
Additionally, the growing adoption of bracing products for preventive and post-operative patient care, and strong presence of several key players in the country with its orthobiologic products in pipeline are likely to contribute towards the dominant market position.
The prominent players operating in this market includes Arthrex (US), Stryker Corporation (US), Smith & Nephew (UK), Johnson & Johnson (DePuy Synthes) (US), Zimmer Biomet (US), CONMED Corporation (US), Integra LifeSciences Corporation (US), LimaCorporate (Italy), FH ORTHOPEDICS S.A.S. (France), Evolutis India Pvt Ltd. (India), DJO Global (US), 3S Ortho (France), Breg, Inc. (US), GPC Medical Ltd. (India), and Parcus Medical (US).
Smith & Nephew is one of the leading global producers of advanced wound management, arthroscopy, trauma and clinical therapy, and orthopedic reconstruction products. The company operates in more than 100 countries across the Americas, Africa, Asia, Australasia, Europe, and the Middle East. The company’s geographic presence is divided into established and emerging markets, where the established markets consist of the US, Canada, Australia, Europe, Japan, and New Zealand.
The company has an advanced integrated deep global distribution network in the shoulder arthroscopic product category. The establishment of a direct sales model has accelerated the Smith & Nephew product platform strategy, firming the company’s position in the orthopedics space. The continuous focus of the company towards the launch of various novel products such as bioabsorbable sutures offers a great opportunity for the company to expand its rotator cuff tear treatment product portfolio. With the execution of clinical trials for Regeneten, a bioinductive collagen for rotator cuff tear, and receiving the CE mark approval in Europe, the company has enhanced its capabilities in developing biologics for rotator cuff tear treatment.
Scope of the Rotator Cuff Injury Treatment Industry
|
Report Metric |
Details |
|
Market Revenue Size in 2021 |
$989.3 million |
|
Projected Revenue Size by 2026 |
$1,244.2 million |
|
Industry Growth Rate |
Poised to Grow at a CAGR of 4.7% |
|
Market Driver |
Rising number of sports-related shoulder injuries |
|
Market Opportunity |
Novel orthobiologics in pipeline |
This research report categorizes the global rotator cuff injury treatment market to forecast revenue and analyze trends in each of the following submarkets:
By Treatment
-
Surgical treatment or curative treatment
- Arthroscopy (tendon repair)
-
Traditional/Open surgeries
- Shoulder Replacement Surgery
- Open Tendon Repair or Transfer
-
Physiotherapy or Palliative treatment
- Orthopedic braces
- Cold compression therapy
-
Pharmaceutical drugs or preventive treatment
- Anti-inflammatory drugs & pain killers
- Injections
- Orthobiologics
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Recent Developments of the Rotator Cuff Injury Treatment Industry
- In 2021, Stryker announced the acquisition of OrthoSpace, Ltd., with an aim to enlarge its sports medicine product offerings, including surgical products for rotator cuff tear treatment.
- In 2021, DePuy Synthes announced the launch of Inhance, a fully integrated shoulder arthroplasty system.
- In 2021, Smith & Nephew announced the launch of a knotless suture, Healicoil, used during the arthroscopic treatment of rotator cuff rupture.
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the rotator cuff injury treatment market?
The rotator cuff injury treatment market boasts a total revenue value of $1,244.2 million by 2026.
What is the estimated growth rate (CAGR) of the rotator cuff injury treatment market?
The global rotator cuff injury treatment market has an estimated compound annual growth rate (CAGR) of 4.7% and a revenue size in the region of $989.3 million in 2021.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst

TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 10)
1.1 OBJECTIVES OF THE STUDY
1.2 ROTATOR CUFF INJURY TREATMENT INDUSTRY DEFINITION
1.2.1 INCLUSIONS & EXCLUSIONS OF THE STUDY
1.3 MARKET SCOPE
1.3.1 MARKETS COVERED
1.3.2 YEARS CONSIDERED FOR THE STUDY
1.4 CURRENCY USED FOR THE STUDY
1.5 MAJOR MARKET STAKEHOLDERS
2 RESEARCH METHODOLOGY (Page No. - 13)
2.1 RESEARCH DATA
2.1.1 SECONDARY DATA
2.1.1.1 Secondary sources
2.1.2 PRIMARY DATA
2.2 MARKET ESTIMATION METHODOLOGY
2.2.1 REVENUE MAPPING-BASED MARKET ESTIMATION
2.2.2 TREATMENT USAGE-BASED MARKET ESTIMATION
2.2.3 PRIMARY RESEARCH VALIDATION
2.3 DATA TRIANGULATION
2.4 RESEARCH ASSUMPTIONS
2.5 RESEARCH LIMITATIONS
3 EXECUTIVE SUMMARY (Page No. - 22)
3.1 ROTATOR CUFF INJURY TREATMENT MARKET
3.2 GLOBAL MARKET, BY TYPE
4 MARKET OVERVIEW (Page No. - 23)
4.1 INTRODUCTION
4.2 MARKET DYNAMICS
4.2.1 INDUSTRY GROWTH OPPORTUNITIES
4.2.1.1 Rising number of sports-related shoulder injuries
4.2.1.2 Rising technological advancements in rotator cuff rupture devices
4.2.1.3 Growing public-private funding grants and educational awareness initiatives
4.2.1.4 Increasing risk of osteoarthritis in the geriatric population
4.2.1.5 Growing developments in orthobiologics
4.2.2 BURNING ISSUES AND CHALLENGES
4.2.2.1 High costs associated with arthroscopic surgery instruments
4.2.2.2 High failure rates of rotator cuff treatment
4.2.2.3 Shortage of trained orthopedic professionals
4.2.3 COVID-19 SPECIFIC TRENDS
4.3 REGULATORY LANDSCAPE
4.3.1 NORTH AMERICA
4.3.1.1 US
4.3.1.2 Canada
4.3.2 EUROPE
4.3.3 ASIA PACIFIC
4.3.3.1 India
4.3.3.2 Japan
4.3.3.3 China
4.3.3.4 India
4.4 REIMBURSEMENT SCENARIO
4.5 PRICING TREND ANALYSIS
4.6 CLINICAL RESEARCH PIPELINE
4.6.1 ROTATOR CUFF REPAIR TREATMENT
4.7 PATENT ANALYSIS
5 ROTATOR CUFF INJURY TREATMENT MARKET, BY TYPE (Page No. - 46)
5.1 INTRODUCTION
5.2 SURGICAL OR CURATIVE TREATMENT
5.2.1 ARTHROSCOPY (TENDON REPAIR)
5.2.2 TRADITIONAL/OPEN SURGERIES
5.2.2.1 Shoulder replacement surgeries
5.2.2.2 Open tendon repair/transfer
5.3 PHYSIOTHERAPY/PALLIATIVE TREATMENT
5.3.1 ORTHOPEDIC BRACES
5.3.2 COLD COMPRESSION
5.4 PHARMACEUTICAL DRUGS/SYMPTOMATIC OR PREVENTIVE TREATMENT
5.4.1 ANTI-INFLAMMATORY DRUGS AND PAIN KILLERS
5.4.2 INJECTIONS
5.5 ORTHOBIOLOGICS
6 ROTATOR CUFF INJURY TREATMENT MARKET, BY REGION (Page No. - 52)
6.1 INTRODUCTION
6.2 NORTH AMERICA
6.3 EUROPE
6.4 ASIA PACIFIC
6.5 LATIN AMERICA
6.6 MIDDLE EAST & AFRICA
7 COMPETITIVE LANDSCAPE (Page No. - 56)
7.1 OVERVIEW
7.1.1 KEY PLAYER STRATEGIES
7.2 LIST OF KEY PLAYERS OPERATING IN EACH TREATMENT SPACE
7.2.1 PRODUCT PORTFOLIO/DEVELOPMENT PIPELINE
7.2.2 KEY STRATEGIES/RIGHT TO WIN
7.3 COMPETITIVE SCENARIO
7.3.1 ROTATOR CUFF INJURY TREATMENT INDUSTRY: PRODUCT LAUNCHES & REGULATORY APPROVALS
7.3.2 MARKET: DEALS
7.3.3 MARKET: OTHER DEVELOPMENTS
7.3.4 COMPETITIVE LEADERSHIP MAPPING
7.3.5 STARS
7.3.6 PERVASIVE PLAYERS
7.3.7 EMERGING LEADERS
7.3.8 PARTICIPANTS
7.4 FOOTPRINT ANALYSIS OF THE TOP PLAYERS IN THE GLOBAL MARKET
7.4.1 TREATMENT PRODUCT AND REGIONAL FOOTPRINT ANALYSIS OF THE TOP PLAYERS IN THE MARKET
8 COMPANY PROFILES (Page No. - 67)
8.1 KEY PLAYERS
8.1.1 STRYKER CORPORATION
8.1.1.1 Business overview
8.1.1.2 Products offered
8.1.1.3 Recent developments
8.1.1.4 MnM view
8.1.2 JOHNSON & JOHNSON
8.1.2.1 Business overview
8.1.2.2 Products offered
8.1.2.3 Recent developments
8.1.2.4 MnM view
8.1.3 SMITH & NEPHEW
8.1.3.1 Business overview
8.1.3.2 Products offered
8.1.3.3 Recent developments
8.1.3.4 MNM view
8.1.4 ARTHREX
8.1.4.1 Business overview
8.1.4.2 Products offered
8.1.4.3 Recent developments
8.1.5 ZIMMER BIOMET
8.1.5.1 Business overview
8.1.5.2 Products offered
8.1.6 INTEGRA LIFESCIENCES
8.1.6.1 Business Overview
8.1.6.2 Products offered
8.1.6.3 Recent developments
8.1.7 LIMACORPORATE S.P.A
8.1.7.1 Business Overview
8.1.7.2 Products offered
8.1.7.3 Recent developments
8.1.8 DJO GLOBAL, INC.
8.1.8.1 Business overview
8.1.8.2 Products offered
8.1.8.3 Recent developments
8.1.9 EVOLUTIS
8.1.9.1 Business overview
8.1.9.2 Products offered
8.1.10 GROUP FH ORTHO
8.1.10.1 Business Overview
8.1.10.2 Products offered
8.1.10.3 Recent developments
8.1.11 CONMED CORPORATION
8.1.11.1 Business overview
8.1.11.2 Products offered
8.1.12 3S ORTHO
8.1.12.1 Business overview
8.1.12.2 Products offered
8.1.13 ANIKA THERAPEUTICS, INC. (PARCUS MEDICAL)
8.1.13.1 Business overview
8.1.13.2 Products offered
8.1.13.3 Recent developments
8.1.14 GPC MEDICAL LTD.
8.1.14.1 Business overview
8.1.14.2 Products offered
8.1.15 BREG, INC.
8.1.15.1 Business overview
8.1.15.2 Products offered
8.1.15.3 Recent developments
9 APPENDIX (Page No. - 104)
9.1 DISCUSSION GUIDE
9.2 KNOWLEDGE STORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
9.3 RELATED REPORTS
9.4 AVAILABLE CUSTOMIZATIONS
LIST OF TABLES (30 Tables)
TABLE 1 NUMBER OF PERSONS AGED 65 YEARS OR OVER BY GEOGRAPHIC REGION (2019 VS. 2050)
TABLE 2 US FDA: MEDICAL DEVICE CLASSIFICATION
TABLE 3 US: MEDICAL DEVICE REGULATORY APPROVAL PROCESS
TABLE 4 CANADA: MEDICAL DEVICE REGULATORY APPROVAL PROCESS
TABLE 5 JAPAN: MEDICAL DEVICE CLASSIFICATION UNDER PMDA
TABLE 6 CHINA: CLASSIFICATION OF MEDICAL DEVICES
TABLE 7 REIMBURSEMENT CODES FOR PHYSICIAN SERVICES (AS OF 2020)
TABLE 8 REIMBURSEMENT CODES FOR PHYSICIAN SERVICES (AS OF 2020)
TABLE 9 AVERAGE PRICE OF ROTATOR CUFF TREATMENT INSTRUMENTS, BY REGION COUNTRY, 2020 (USD)
TABLE 10 ROTATOR CUFF REPAIR: INDICATIVE LIST OF ONGOING CLINICAL TRIALS (AS OF JANUARY 2018)
TABLE 11 KEY PRODUCT LAUNCHES & REGULATORY APPROVALS, JANUARY 2018–OCTOBER 2021
TABLE 12 KEY DEALS, JANUARY 2018–OCTOBER 2021
TABLE 13 OTHER KEY DEVELOPMENTS, JANUARY 2018–OCTOBER 2021
TABLE 14 TREATMENT PRODUCT FOOTPRINT OF COMPANIES
TABLE 15 REGIONAL FOOTPRINT OF COMPANIES
TABLE 16 STRYKER CORPORATION: BUSINESS OVERVIEW
TABLE 17 JOHNSON & JOHNSON: BUSINESS OVERVIEW
TABLE 18 SMITH & NEPHEW: BUSINESS OVERVIEW
TABLE 19 ARTHREX: BUSINESS OVERVIEW
TABLE 20 ZIMMER BIOMET: BUSINESS OVERVIEW
TABLE 21 INTEGRA LIFESCIENCES: BUSINESS OVERVIEW
TABLE 22 LIMACORPORATE: BUSINESS OVERVIEW
TABLE 23 DJO GLOBAL, INC.: BUSINESS OVERVIEW
TABLE 24 EVOLUTIS: BUSINESS OVERVIEW
TABLE 25 GROUP FH ORTHO: BUSINESS OVERVIEW
TABLE 26 CONMED CORPORATION: BUSINESS OVERVIEW
TABLE 27 3S ORTHO: BUSINESS OVERVIEW
TABLE 28 ANIKA THERAPEUTICS: BUSINESS OVERVIEW
TABLE 29 GPC MEDICAL LTD.: BUSINESS OVERVIEW
TABLE 30 BREG, INC.: BUSINESS OVERVIEW
LIST OF FIGURES (23 Figures)
FIGURE 1 RESEARCH DESIGN
FIGURE 2 BREAKDOWN OF PRIMARIES: ROTATOR CUFF INJURY TREATMENT MARKET
FIGURE 3 RESEARCH METHODOLOGY: HYPOTHESIS BUILDING
FIGURE 4 ROTATOR CUFF INJURY TREATMENT INDUSTRY SIZE ESTIMATION BASED ON TREATMENT UTILIZATION METHODOLOGY
FIGURE 5 ROTATOR CUFF INJURY TREATMENT INDUSTRY SIZE ESTIMATION BASED ON REVENUE MAPPING METHODOLOGY
FIGURE 6 DATA TRIANGULATION METHODOLOGY
FIGURE 7 INJURIES CAUSED BY VARIOUS SPORTS ACTIVITIES IN THE US PER 1000 INHABITANATS (2020)
FIGURE 8 CANADA: REGULATORY APPROVAL PROCESS FOR MEDICAL DEVICES (MDR)
FIGURE 9 EUROPE: REGULATORY APPROVAL PROCESS FOR MEDICAL DEVICES (MDR)
FIGURE 10 TOP 10 PATENT APPLICANTS FOR ROTATOR CUFF REPAIR (JANUARY 2011 TO OCTOBER 2021)
FIGURE 11 NUMBER OF GRANTED PATENTS AND PATENT APPLICATIONS (JANUARY 2011 TO OCTOBER 2021)
FIGURE 12 PATENT DOCUMENTS FOR ROTATOR CUFF REPAIR, BY LEGAL STATUS (JANUARY 2011 TO OCTOBER 2021)
FIGURE 13 REGIONAL ANALYSIS OF PATENTS GRANTED FOR ROTATOR CUFF REPAIR, 2020
FIGURE 14 ROTATOR CUFF INJURY TREATMENT MARKET: COMPETITIVE LEADERSHIP MAPPING, 2020
FIGURE 15 STRYKER CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 16 JOHNSON & JOHNSON: COMPANY SNAPSHOT (2020)
FIGURE 17 SMITH & NEPHEW: COMPANY SNAPSHOT (2020)
FIGURE 18 ZIMMER BIOMET: COMPANY SNAPSHOT (2020)
FIGURE 19 INTEGRA LIFESCIENCES: COMPANY SNAPSHOT (2020)
FIGURE 20 LIMACORPORATE: COMPANY SNAPSHOT (2020)
FIGURE 21 DJO GLOBAL, INC.: COMPANY SNAPSHOT (2020)
FIGURE 22 CONMED CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 23 ANIKA THERAPEUTICS, INC.: COMPANY SNAPSHOT (2020)
The study involved four major activities in estimating the current size of the rotator cuff injury treatment market. Exhaustive secondary research was carried out to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Top-down and bottom-up approaches were employed to estimate the complete market size. Thereafter, market breakdown and data triangulation procedures were used to estimate the size of segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources, such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, regulatory bodies, and publications from government sources such as World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), American Academy Of Orthopaedic Surgeons (AAOS), American Orthopaedic Society For Sports Medicine (AOSSM), American Trauma Society (ATS were referred to identify and collect information for the global market study.
Primary Research
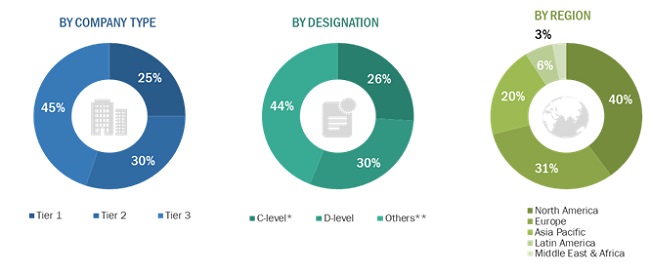
The global market comprises several stakeholders such as manufacturers Of rotator cuff injury treatment devices and pharmaceutical drugs, research, and consulting firms. The demand side of this market is characterized by the increase in sports-related shoulder injuries, technological advancements in the arthroscopic treatment of rotator cuff tear, presence of well-established players in the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information. The following is the breakdown of primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the rotator cuff injury treatment market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and markets have been identified through extensive secondary research
- The industry’s supply chain and market size, in terms of value, have been determined through primary and secondary research
- All percentage shares splits, and breakdowns have been determined using secondary sources and verified through primary sources
Market Size Estimation Based On Treatment Utilization Methodology

Market Size Estimation Based On Revenue Mapping Methodology
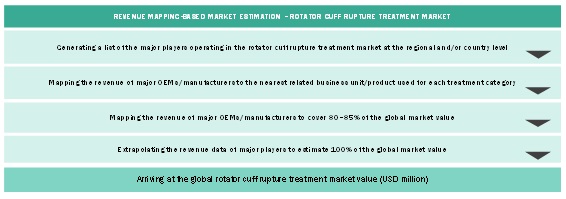
Data Triangulation
After arriving at the overall size of the global market through the above-mentioned methodology, this market was split into several segments and subsegments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact market value data for the key segments and subsegments. The extrapolated market data was triangulated by studying various macro indicators and regional trends from both demand- and supply-side participants.
Report Objectives
- To define, describe, and forecast the global rotator cuff injury treatment market on the basis of product, modality, usage, disease indication, and region.
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micromarkets1 with respect to individual growth trends, future prospects, and contributions to the overall market
- To analyze the opportunities in the market for key stakeholders and provide details of the competitive landscape for major market leaders
- To forecast the size of the market segments with respect to five main regions, namely, North America (the US and Canada), Europe (Germany, the UK, France, Italy, Spain, and Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, and Rest of Asia Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa
- To profile the key market players and comprehensively analyze their market shares and core competencies2
- To track and analyze competitive developments such as mergers and acquisitions, new product developments, partnerships, agreements, collaborations, and expansions in the global market .
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the global market report
Product Analysis
- Product Matrix, which gives a detailed comparison of the product portfolios of the top five global players.
Company Information
- Detailed analysis and profiling of additional market players (up to 5 OEMs)
Geographic Analysis
- Further breakdown of the Rest of Europe market into Belgium, Austria, Denmark, Greece, Poland, and Russia, among other countries
- Further breakdown of the Rest of Asia Pacific market into New Zealand, Vietnam, Philippines, Singapore, Malaysia, Thailand, and Indonesia, among other countries
- Further breakdown of the Rest of Latin America market into Argentina, and Colombia, among other countries.



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Rotator Cuff Injury Treatment Market
What is the Worldwide Rotator Cuff Injury Treatment Industry is Expected to Reach by 2026 ?
Can you share the detailed data on the recent developments in the global Rotator Cuff Injury Treatment Market?
Which of the key players holds how much percent share of the global Rotator Cuff Injury Treatment Market?