Mycoplasma Testing Market by Product & Service (Assays, Kits, Reagents), Technique (NAT, ELISA, Staining), Application (Cell Line, End of Production Testing), End User (Biopharmaceutical, Cell Banks, CROs) & Region - Global Forecast to 2025
Market Growth Outlook Summary
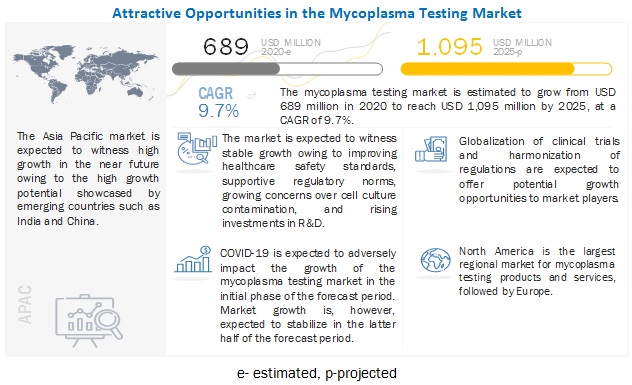
The global mycoplasma testing market growth forecasted to transform from $689 million in 2020 to $1,095 million by 2025, driven by a CAGR of 9.7%. The global market's growth is driven by factors such as the growing concerns over cell culture contamination, growth in the pharmaceutical and biotechnology industries, and rising pharmaceutical R & D activities and investments.
To know about the assumptions considered for the study, Request for Free Sample Report
Mycoplasma Testing Market Dynamics
Driver: Growing concerns over cell culture contamination
Cell culture contamination is the most common problem encountered in cell culture laboratories. Cell culture contaminants are divided into two main categories, namely, chemical contaminants and biological contaminants. Chemical contaminants include impurities in media, sera, water, endotoxins, plasticizers, and detergents, while biological contaminants include bacteria, mold, yeast, viruses, and mycoplasma as cross-contamination by other cell lines. For contamination detection, various tests, including mycoplasma testing, are used.
Opportunity: Globalization of clinical trials and R&D and harmonization of regulations resulting in increased outsourcing
The primary reasons pharmaceutical and biopharmaceutical companies are opting for outsourcing clinical trials, and R&D are cost efficiency, easy patient recruitment, reduction in operating costs, and less-stringent regulatory frameworks than the US and Western Europe. Besides reducing costs, sponsors attribute improved quality and reduction in the time to market as major drivers for outsourcing their business functions to CROs, CDMOs, and CMOs. Hence, many pharmaceutical and biotechnology companies and academic institutes opt to outsource functions like manufacturing, clinical trial management, R&D, and drug discovery to CROs.
Challenge: High degree of consolidation acts as an entry barrier for new entrants
The top players in this market are large and well-established and enjoy a high degree of brand loyalty. New entrants in this market compete with existing players and set themselves apart by developing innovative product offerings. The high degree of consolidation acts as a major entry barrier for new entrants, which in turn, is expected to restrict investments and thus slow down the growth potential of this market.
Assays, Kits, & Reagents products segment accounted for the largest share of the mycoplasma testing industry, by product & service
The global market is segmented into assays, kits, & reagents; instruments; and services based on product & service. In 2019, the assays, kits, & reagents segment accounted for this market's largest share. The frequent purchase of these consumables as compared to instruments and the increasing use of kit-based techniques for mycoplasma testing are the important factors driving the growth of this market segment.
Cell line testing segment accounted for the largest share in the mycoplasma testing industry, by application
Based on application, the global market is segmented into cell line testing, virus testing, and end-of-production cell testing. In 2019, the cell line testing segment accounted for the largest share of this market. Factors such as the rapid growth in the biopharmaceutical industry and the increasing demand for monoclonal antibodies drive this market segment's growth.
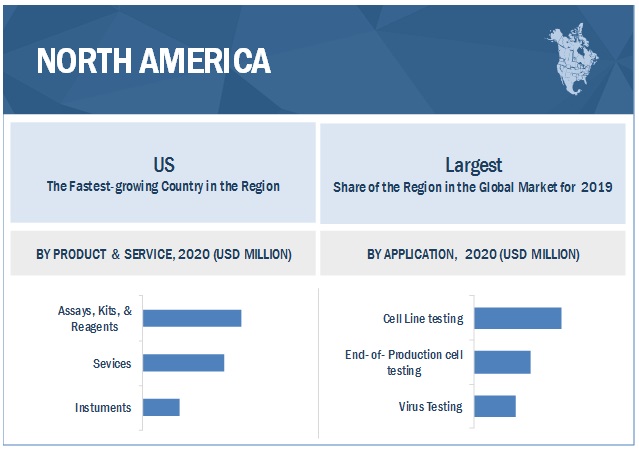
North America is the largest regional market for mycoplasma testing industry
The global market is segmented into North America, Europe, the Asia Pacific, and the Rest of the World. In 2019, North America accounted for the largest share of the global market. The large share of this regional segment can primarily be attributed to the growth in biotechnology and pharmaceutical industries, the high adoption rate of novel technologies, and the large number of life science research studies conducted in this region.
The major players operating in this market are Thermo Fisher Scientific, Inc. (US), Charles River Laboratories International, Inc. (US), Lonza Group Ltd. (Switzerland), Merck KGaA (Germany), Roche Diagnostics (Switzerland), American Type Culture Collection (US), Bionique Testing Laboratories, Inc. (US), InvivoGen (US), PromoCell GmbH (Germany), Biological Industries Israel Beit Haemek Ltd. (Israel), Creative Bioarray (US), Mycoplasma Experience (UK), Norgen Biotek Corp. (Canada), Agilent Technologies (US), Biotools B & M Labs, S.A. (Spain), among others.
Scope of the Mycoplasma Testing Industry
|
Report Metric |
Details |
|
Market Revenue Size in 2020 |
$689 million |
|
Projected Revenue Size by 2025 |
$1,095 million |
|
Industry Growth Rate |
Poised to Grow at a CAGR of 9.7% |
|
Market Driver |
Growing concerns over cell culture contamination |
|
Market Opportunity |
Globalization of clinical trials and R&D and harmonization of regulations resulting in increased outsourcing |
This report categorizes the mycoplasma testing market to forecast revenue and analyze trends in each of the following submarkets:
By Product & Service
-
Assays, Kits, & Reagents
- NAT assays, kits, & reagents
- ELISA assays, kits, & reagents
- Elimination Kits
- Stains
- Other assays, kits, & reagents
- Instruments
- Services
By Technique
- NAT
- ELISA
- Staining
- Other technique
By Application
- Cell line testing
- Virus Testing
- End- of- Production cell testing
By End User
- Pharmaceutical & Biotechnology Companies
- Cell Banks & Laboratories
- Contract Research Organizations
- Academic Research Institutes
By Region
-
North America
- US
- Canada
-
Europe
- UK
- Germany
- France
- Rest of Europe
-
Asia Pacific
- Japan
- China
- Australia
- Rest of Asia Pacific
- Rest of the World
Recent Developments of Mycoplasma Testing Industry:
- In July 2020, Roche Diagnostics launched Cobas 6800, a fully automated system in India.
- In July 2020, Creative Bioarray launched a full range of cell-based services
- In January 2020, Charles River Laboratories (US) acquired HemaCare Corporation (US)
Frequently Asked Questions (FAQ):
What are the recent trends affecting the mycoplasma testing market?
Recent trends affecting the mycoplasma testing market are growing concerns over cell culture contamination, growth in the pharmaceutical and biotechnology industries, and rising pharmaceutical R&D activities and investments.
Who are the key players in the market, and how intense is the competition?
The key players in this market are: Thermo Fisher Scientific, Inc. (US), Charles River Laboratories International, Inc. (US), Lonza Group Ltd. (Switzerland), Merck KGaA (Germany), Roche Diagnostics (Switzerland), American Type Culture Collection (US), Bionique Testing Laboratories, Inc. (US), InvivoGen (US), PromoCell GmbH (Germany), Biological Industries Israel Beit Haemek Ltd. (Israel), Creative Bioarray (US), Mycoplasma Experience (UK), Norgen Biotek Corp. (Canada), Agilent Technologies (US), Biotools B & M Labs, S.A. (Spain), Eurofins Scientific (Luxembourg), GeneCopoeia, Inc. (US), GenBio (Canada), Minerva Biolabs GmbH (Germany), Meridian Bioscience, Inc. (US), Sartorius AG (Germany), Savyon Diagnostics (Israel), Nelson Laboratories Fairfield, Inc. (US), Clongen Laboratories, LLC (US), and ScienCell Research Laboratories, Inc. (Canada).
What are the key applications of mycoplasma testing products?
These products are used in various applications like cell line testing, virus testing, and end-of-production cell testing.
Which region is lucrative for the mycoplasma testing market?
The emerging economies in Asia Pacific region like China, Japan, India will be the lucrative markets for mycoplasma testing products.
What are the key end users of mycoplasma testing products & services?
The end users for mycoplasma testing products & services are pharmaceutical & biotechnology companies; contract research organizations; academic research institutes, and cell banks & laboratories. .
To speak to our analyst for a discussion on the above findings, click Speak to Analyst

TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 24)
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
FIGURE 1 INCLUSIONS & EXCLUSIONS
1.2.1 MARKETS COVERED
FIGURE 2 MYCOPLASMA TESTING MARKET SEGMENTATION
1.2.2 YEARS CONSIDERED FOR THE STUDY
1.3 CURRENCY
1.4 LIMITATIONS
1.5 STAKEHOLDERS
1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY (Page No. - 29)
2.1 RESEARCH DATA
FIGURE 3 RESEARCH DESIGN
2.1.1 SECONDARY DATA
2.1.1.1 Key data from secondary sources
2.1.2 PRIMARY DATA
FIGURE 4 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
2.2 MARKET SIZE ESTIMATION
2.2.1 BOTTOM-UP APPROACH
FIGURE 5 GLOBAL MARKET: BOTTOM-UP APPROACH
2.2.2 TOP-DOWN APPROACH
FIGURE 6 GLOBAL MARKET: TOP-DOWN APPROACH
2.3 MARKET BREAKDOWN & DATA TRIANGULATION
FIGURE 7 DATA TRIANGULATION METHODOLOGY
2.4 ASSUMPTIONS FOR THE STUDY
3 EXECUTIVE SUMMARY (Page No. - 36)
FIGURE 8 GLOBAL MARKET, BY PRODUCT & SERVICE, 2020 VS. 2025 (USD MILLION)
FIGURE 9 GLOBAL MARKET, BY APPLICATION, 2020 VS. 2025 (USD MILLION)
FIGURE 10 GLOBAL MARKET, BY END USER, 2020 VS. 2025 (USD MILLION)
FIGURE 11 GLOBAL MARKET, BY REGION, 2020 VS. 2025 (USD MILLION)
4 PREMIUM INSIGHTS (Page No. - 39)
4.1 MYCOPLASMA TESTING MARKET OVERVIEW
FIGURE 12 RISING INVESTMENTS IN PHARMACEUTICAL R&D TO DRIVE MARKET GROWTH
4.2 MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2020 VS. 2025 (USD MILLION)
FIGURE 13 NAT ASSAYS, KITS, & REAGENTS SEGMENT TO COMMAND THE LARGEST MARKET SHARE IN 2019
4.3 GLOBAL MARKET, BY TECHNIQUE, 2020 VS. 2025
FIGURE 14 GROWING ADOPTION OF NAT IN EMERGING ECONOMIES TO DRIVE MARKET GROWTH
4.4 GLOBAL MARKET, BY REGION, 2020 VS. 2025
FIGURE 15 ASIA PACIFIC MARKET TO GROW AT THE HIGHEST CAGR DURING THE FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 42)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
FIGURE 16 MYCOPLASMA TESTING MARKET: DRIVERS, OPPORTUNITIES, AND CHALLENGES
5.2.1 DRIVERS
5.2.1.1 Growing concerns over cell culture contamination
5.2.1.2 Growth in pharmaceutical and biotechnology industries
TABLE 1 TOP BIOTECHNOLOGY INVESTMENT DEALS, 2019–2020
5.2.1.3 Rising pharmaceutical R&D activities and investments
FIGURE 17 INCIDENCE OF THE TOP 10 FATAL CONDITIONS GLOBALLY, 2019 (%)
TABLE 2 RISING CANCER INCIDENCE, BY COUNTRY/REGION, 2018 VS. 2025
FIGURE 18 R&D SPENDING BY PHRMA MEMBER-COMPANIES, 2001–2019 (USD BILLION)
TABLE 3 PHARMACEUTICAL R&D SPENDING, BY COMPANY, 2018
5.2.2 OPPORTUNITIES
5.2.2.1 Globalization of clinical trials and R&D and harmonization of regulations resulting in increased outsourcing
5.2.2.2 Emerging economies
5.2.3 CHALLENGES
5.2.3.1 High degree of consolidation acts as an entry barrier for new entrants
5.3 IMPACT OF COVID-19 ON THE GLOBAL MARKET
5.4 VALUE CHAIN ANALYSIS
FIGURE 19 VALUE CHAIN ANALYSIS OF THE GLOBAL MARKET
FIGURE 20 RESTRAINTS IN THE VALUE CHAIN, BY STAGE
5.5 SUPPLY CHAIN ANALYSIS
FIGURE 21 SUPPLY CHAIN ANALYSIS OF THE GLOBAL MARKET
5.6 ECOSYSTEM ANALYSIS
FIGURE 22 ECOSYSTEM ANALYSIS OF THE GLOBAL MARKET
6 MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE (Page No. - 51)
6.1 INTRODUCTION
TABLE 4 AVERAGE SELLING PRICE
TABLE 5 GLOBAL MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
6.2 ASSAYS, KITS, & REAGENTS
6.2.1 INCREASING ADOPTION OF NAT TO DRIVE MARKET GROWTH
TABLE 6 MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 7 MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.2.2 NAT ASSAYS, KITS, & REAGENTS
TABLE 8 NAT ASSAYS, KITS, & REAGENTS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.2.3 ENZYME-LINKED IMMUNOSORBENT ASSAYS, KITS, & REAGENTS
TABLE 9 ENZYME-LINKED IMMUNOSORBENT ASSAYS, KITS, & REAGENTS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.2.4 ELIMINATION KITS
TABLE 10 ELIMINATION KITS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.2.5 STAINS
TABLE 11 MYCOPLASMA TESTING STAINS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.2.6 OTHER ASSAYS, KITS, & REAGENTS
TABLE 12 OTHER ASSAYS, KITS, & REAGENTS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.3 INSTRUMENTS
6.3.1 DEMAND FOR FULLY AUTOMATED INSTRUMENTS IS INCREASING IN RESEARCH APPLICATIONS
TABLE 13 MYCOPLASMA TESTING INSTRUMENTS MARKET, BY REGION, 2018–2025 (USD MILLION)
6.4 SERVICES
6.4.1 LACK OF INFRASTRUCTURE AND EXPERTISE HAVE DRIVEN RELIANCE ON SERVICE PROVIDERS
TABLE 14 MYCOPLASMA TESTING SERVICES MARKET, BY REGION, 2018–2025 (USD MILLION)
7 MYCOPLASMA TESTING MARKET, BY TECHNIQUE (Page No. - 59)
7.1 INTRODUCTION
TABLE 15 GLOBAL MARKET, BY TECHNIQUE, 2018–2025 (USD MILLION)
7.2 NUCLEIC ACID TESTING
7.2.1 NAT SEGMENT DOMINATED THE MARKET IN 2019
TABLE 16 NAT-BASED MYCOPLASMA-TESTING MARKET, BY REGION, 2018–2025 (USD MILLION)
7.3 ENZYME-LINKED IMMUNOSORBENT ASSAY
7.3.1 ELISA IS WIDELY USED FOR MYCOPLASMA DETECTION IN RESEARCH APPLICATIONS
TABLE 17 ELISA-BASED MYCOPLASMA-TESTING MARKET, BY REGION, 2018–2025 (USD MILLION)
7.4 STAINING
7.4.1 STAINING OF CULTURES CAN PROVIDE RAPID RESULTS
TABLE 18 STAINING-BASED MYCOPLASMA-TESTING MARKET, BY REGION, 2018–2025 (USD MILLION)
7.5 OTHER TECHNIQUES
TABLE 19 OTHER TECHNIQUES-BASED MYCOPLASMA-TESTING MARKET, BY REGION, 2018–2025 (USD MILLION)
8 MYCOPLASMA TESTING MARKET, BY APPLICATION (Page No. - 64)
8.1 INTRODUCTION
TABLE 20 GLOBAL MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
8.2 CELL LINE TESTING
8.2.1 RAPID GROWTH IN THE BIOPHARMACEUTICAL INDUSTRY TO DRIVE THE DEMAND FOR CELL LINE TESTING
TABLE 21 GLOBAL MARKET FOR CELL LINE TESTING, BY REGION, 2018–2025 (USD MILLION)
TABLE 22 NORTH AMERICA: MARKET FOR CELL LINE TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 23 EUROPE: MARKET FOR CELL LINE TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 24 ASIA PACIFIC: MARKET FOR CELL LINE TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
8.3 VIRUS TESTING
8.3.1 RISING DEMAND FOR VACCINES TO DRIVE MARKET GROWTH
TABLE 25 KEY VIRUS TESTING PRODUCTS/SERVICES OFFERED BY MARKET PLAYERS
TABLE 26 GLOBAL MARKET FOR VIRUS TESTING, BY REGION, 2018–2025 (USD MILLION)
TABLE 27 NORTH AMERICA: MARKET FOR VIRUS TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 28 EUROPE: MARKET FOR VIRUS TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 29 ASIA PACIFIC: MARKET FOR VIRUS TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
8.4 END-OF-PRODUCTION CELL TESTING
8.4.1 GROWING DEMAND FOR BIOLOGICS TO DRIVE MARKET GROWTH
TABLE 30 GLOBAL MARKET FOR END-OF-PRODUCTION CELL TESTING, BY REGION, 2018–2025 (USD MILLION)
TABLE 31 NORTH AMERICA: MARKET FOR END-OF-PRODUCTION CELL TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 32 EUROPE: MARKET FOR END-OF-PRODUCTION CELL TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 33 ASIA PACIFIC: MARKET FOR END-OF-PRODUCTION CELL TESTING, BY COUNTRY, 2018–2025 (USD MILLION)
9 MYCOPLASMA TESTING MARKET, BY END USER (Page No. - 72)
9.1 INTRODUCTION
TABLE 34 GLOBAL MARKET, BY END USER, 2018–2025 (USD MILLION)
9.2 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
9.2.1 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES FORM THE LARGEST END-USER SEGMENT IN THE GLOBAL MARKET
FIGURE 23 R&D INVESTMENTS IN THE PHARMACEUTICAL INDUSTRY, 2012–2026 (USD BILLION)
TABLE 35 PHARMACEUTICAL PATENT GRANTS, 2017 VS. 2019, BY COUNTRY
TABLE 36 GLOBAL MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2018–2025 (USD MILLION)
9.3 CONTRACT RESEARCH ORGANIZATIONS
9.3.1 GROWTH IN OUTSOURCING TO DRIVE THE GROWTH OF THIS END-USER SEGMENT
TABLE 37 GLOBAL MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY REGION, 2018–2025 (USD MILLION)
9.4 ACADEMIC & RESEARCH INSTITUTES
9.4.1 ACADEMIC AND RESEARCH INSTITUTES PROVIDE VARIOUS MICROBIOLOGY TESTING SERVICES
TABLE 38 GLOBAL MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2018–2025 (USD MILLION)
9.5 CELL BANKS & LABORATORIES
9.5.1 IT IS ESTIMATED THAT ABOUT 5–30% OF THE WORLD'S CELL LINES ARE CONTAMINATED WITH MYCOPLASMAS
TABLE 39 GLOBAL MARKET FOR CELL BANKS & LABORATORIES, BY REGION, 2018–2025 (USD MILLION)
10 MYCOPLASMA TESTING MARKET, BY REGION (Page No. - 79)
10.1 INTRODUCTION
TABLE 40 GLOBAL MARKET, BY REGION, 2018–2025 (USD MILLION)
10.2 NORTH AMERICA
FIGURE 24 NORTH AMERICA: MYCOPLASMA TESTING MARKET SNAPSHOT
TABLE 41 NORTH AMERICA: MARKET, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 42 NORTH AMERICA: MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 43 NORTH AMERICA: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 44 NORTH AMERICA: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 45 NORTH AMERICA: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.2.1 US
10.2.1.1 US is the largest market for mycoplasma testing products and services in North America
TABLE 46 US: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 47 US: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 48 US: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 49 US: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.2.2 CANADA
10.2.2.1 Increasing pharma and biotech R&D investments to drive market growth
TABLE 50 CANADA: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 51 CANADA: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 52 CANADA: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 53 CANADA: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3 EUROPE
TABLE 54 EUROPE: MYCOPLASMA TESTING MARKET, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 55 EUROPE: MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 56 EUROPE: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 57 EUROPE: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 58 EUROPE: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3.1 GERMANY
10.3.1.1 Germany accounted for the largest share of the European market in 2019
TABLE 59 GERMANY: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 60 GERMANY: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 61 GERMANY: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 62 GERMANY: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3.2 UK
10.3.2.1 Growth in the life science industry to support market growth in the UK
TABLE 63 UK: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 64 UK: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 65 UK: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 66 UK: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3.3 FRANCE
10.3.3.1 Increasing investments in life science R&D to support market growth in France
TABLE 67 FRANCE: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 68 FRANCE: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 69 FRANCE: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 70 FRANCE: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3.4 ITALY
10.3.4.1 Italy is one of the leaders in life science research in Europe
TABLE 71 ITALY: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 72 ITALY: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 73 ITALY: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 74 ITALY: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3.5 SPAIN
10.3.5.1 Increasing opportunities for generics and biosimilars manufacturers to support market growth in Spain
TABLE 75 SPAIN: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 76 SPAIN: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 77 SPAIN: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 78 SPAIN: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.3.6 REST OF EUROPE
TABLE 79 ROE: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 80 ROE: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 81 ROE: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 82 ROE: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.4 ASIA PACIFIC
FIGURE 25 APAC: MYCOPLASMA TESTING MARKET SNAPSHOT
TABLE 83 APAC: MARKET, BY COUNTRY, 2018–2025 (USD MILLION)
TABLE 84 APAC: MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 85 APAC: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 86 APAC: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 87 APAC: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.4.1 CHINA
10.4.1.1 China accounted for the largest share of the APAC market in 2019
TABLE 88 CHINA: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 89 CHINA: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 90 CHINA: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 91 CHINA: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.4.2 JAPAN
10.4.2.1 Increasing demand for technologically advanced research products to drive market growth in Japan
TABLE 92 JAPAN: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 93 JAPAN: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 94 JAPAN: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 95 JAPAN: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.4.3 INDIA
10.4.3.1 Growing CRO base in India to support market growth
TABLE 96 INDIA: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 97 INDIA: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 98 INDIA: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 99 INDIA: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.4.4 REST OF ASIA PACIFIC
TABLE 100 ROAPAC: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 101 ROAPAC: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 102 ROAPAC: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 103 ROAPAC: MARKET, BY END USER, 2018–2025 (USD MILLION)
10.5 REST OF THE WORLD
TABLE 104 ROW: MYCOPLASMA TESTING MARKET, BY PRODUCT & SERVICE, 2018–2025 (USD MILLION)
TABLE 105 ROW: MYCOPLASMA TESTING ASSAYS, KITS, & REAGENTS MARKET, BY TYPE, 2018–2025 (USD MILLION)
TABLE 106 ROW: MARKET, BY APPLICATION, 2018–2025 (USD MILLION)
TABLE 107 ROW: MARKET, BY END USER, 2018–2025 (USD MILLION)
11 COMPETITIVE LANDSCAPE (Page No. - 114)
11.1 OVERVIEW
FIGURE 26 KEY DEVELOPMENTS IN THE MYCOPLASMA TESTING MARKET (JANUARY 2017–SEPTEMBER 2020)
11.2 GLOBAL MARKET SHARE ANALYSIS
FIGURE 27 GLOBAL MARKET SHARE ANALYSIS, BY KEY PLAYER, 2019
11.3 COMPANY EVALUATION QUADRANT
11.3.1 DEFINITION AND METHODOLOGY
11.3.2 VENDOR DIVE OVERVIEW
11.3.2.1 Stars
11.3.2.2 Emerging leaders
11.3.2.3 Pervasive
11.3.2.4 Participants
FIGURE 28 GLOBAL MARKET: COMPETITIVE LEADERSHIP MAPPING, 2019
11.4 COMPETITIVE SCENARIO
11.4.1 PRODUCT/SERVICE LAUNCHES
TABLE 108 PRODUCT/SERVICE LAUNCHES & REGULATORY APPROVALS (JANUARY 2017–SEPTEMBER 2020)
11.4.2 PARTNERSHIPS, AGREEMENTS, AND COLLABORATIONS
TABLE 109 PARTNERSHIPS, AGREEMENTS, AND COLLABORATIONS (JANUARY 2017–SEPTEMBER 2020)
11.4.3 ACQUISITIONS
TABLE 110 ACQUISITIONS (JANUARY 2017–SEPTEMBER 2020)
11.4.4 EXPANSIONS
TABLE 111 EXPANSIONS (JANUARY 2017–SEPTEMBER 2020)
12 COMPANY PROFILES (Page No. - 119)
12.1 KEY PLAYERS
(Business overview, Products offered, Recent developments, MNM view)*
12.1.1 MERCK KGAA
FIGURE 29 MERCK KGAA: COMPANY SNAPSHOT (2019)
12.1.2 ROCHE DIAGNOSTICS (A DIVISION OF F. HOFFMAN-LA ROCHE LTD.)
FIGURE 30 F. HOFFMANN-LA ROCHE LTD.: COMPANY SNAPSHOT (2019)
12.1.3 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
FIGURE 31 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.: COMPANY SNAPSHOT (2019)
12.1.4 LONZA GROUP LTD.
FIGURE 32 LONZA GROUP LTD.: COMPANY SNAPSHOT (2019)
12.1.5 THERMO FISHER SCIENTIFIC, INC.
FIGURE 33 THERMO FISHER SCIENTIFIC, INC.: COMPANY SNAPSHOT (2019)
12.1.6 EUROFINS SCIENTIFIC GROUP
FIGURE 34 EUROFINS SCIENTIFIC GROUP: COMPANY SNAPSHOT (2019)
12.1.7 SARTORIUS STEDIM BIOTECH S.A.
FIGURE 35 SARTORIUS STEDIM BIOTECH S.A.: COMPANY SNAPSHOT (2019)
12.1.8 AGILENT TECHNOLOGIES, INC.
FIGURE 36 AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT (2019)
12.1.9 MERIDIAN BIOSCIENCE, INC.
FIGURE 37 MERIDIAN BIOSCIENCE, INC.: COMPANY SNAPSHOT (2019)
12.1.10 AMERICAN TYPE CULTURE COLLECTION
12.2 OTHER PLAYERS
12.2.1 BIONIQUE TESTING LABORATORIES, INC.
12.2.2 INVIVOGEN
12.2.3 PROMOCELL GMBH
12.2.4 BIOLOGICAL INDUSTRIES ISRAEL BEIT HAEMEK LTD.
12.2.5 CLONGEN LABORATORIES, LLC.
12.2.6 CREATIVE BIOARRAY
12.2.7 NELSON LABORATORIES FAIRFIELD, INC. (A PART OF SOTERA HEALTH)
12.2.8 NORGEN BIOTEK CORPORATION
12.2.9 BIOTOOLS B & M LABS, S.A.
12.2.10 GENBIO (A PART OF EXONHIT THERAPEUTICS SA)
12.2.11 GENECOPOEIA, INC.
12.2.12 MINERVA BIOLABS GMBH
12.2.13 MYCOPLASMA EXPERIENCE
12.2.14 SAVYON DIAGNOSTICS
12.2.15 SCIENCELL RESEARCH LABORATORIES
*Business overview, Products offered, Recent developments, MNM view might not be captured in case of unlisted companies.
13 APPENDIX (Page No. - 148)
13.1 DISCUSSION GUIDE
13.2 KNOWLEDGE STORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
13.3 AVAILABLE CUSTOMIZATIONS
13.4 RELATED REPORTS
13.5 AUTHOR DETAILS
This study involved four major activities in estimating the current size of the mycoplasma testing market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate segments and subsegments' market size.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to i identify and collect information for this study.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources were mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify the critical qualitative and quantitative information and assess future prospects.
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the mycoplasma testing market's total size. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry have been identified through extensive secondary research
- The revenues generated by leading players operating in the mycoplasma testing market have been determined through primary and secondary research
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
Global Mycoplasma testing market Size: Top Down Approach

To know about the assumptions considered for the study, Request for Free Sample Report
Data Triangulation
After arriving at the overall market size applying the process mentioned above, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Report Objectives
- To define, describe, and forecast the global mycoplasma testing market based on product & service, technique, application, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (drivers, restraints, opportunities, and challenges)
- To strategically analyze micromarkets with respect to individual growth trends, future prospects, and contributions to the overall market
- To analyze the opportunities in the market for stakeholders and provide details of a competitive landscape for market leaders
- To forecast the size of the global mycoplasma testing market with respect to four main regions (along with countries), namely, North America, Europe, the Asia Pacific, and the Rest of the World
- To profile the key players in the global mycoplasma testing market and comprehensively analyze their core competencies and market shares
- To track and analyze competitive developments such as agreements, partnerships, acquisitions, product launches, and expansions in the global mycoplasma testing market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Country Information
- Mycoplasma testing market size and growth rate estimates for counties in Rest of Europe, the Rest of Asia Pacific and Rest of the World
Company profiles
- Additional five company profiles of players operating in the mycoplasma testing market



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Mycoplasma Testing Market