
Endoscope Reprocessing Market Size, Growth, Share & Trends Analysis
Endoscope Reprocessing Market by Product (AER, HLD & Test Strip, Detergent & Wipe, Endoscope Drying, Storage, & Transport System, Tracking Solution), Endoscope Type (Flexible, Rigid), End User (Hospital, ASC, Specialty Clinic) - Global Forecast to 2030




OVERVIEW

Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The global endoscope reprocessing market is expected to grow from USD 2.71 billion in 2025 to USD 4.24 billion by 2030, registering a CAGR of 9.4% during the forecast period. Market growth is primarily driven by the rising prevalence of gastrointestinal cancers and chronic diseases, which is increasing the volume of endoscopic diagnostic and surgical procedures. The growing emphasis on infection prevention and stricter hygiene standards across healthcare systems has amplified the need for effective endoscope reprocessing solutions. In addition, heightened awareness of endoscope-associated infections (EAIs) and initiatives by global health authorities to strengthen reprocessing guidelines are accelerating adoption. Emerging economies, with rapid improvements in healthcare infrastructure and infection control practices, present significant opportunities for market expansion. Furthermore, the shift toward automated high-level disinfection systems, advancements in reprocessing consumables, and rising hospital investments in centralized sterile processing departments (CSPDs) are expected to further support market growth.
KEY TAKEAWAYS
-
BY PRODUCTThe endoscope reprocessing market is segmented into endoscope reprocessing equipment, endoscope reprocessing consumables, and endoscope tracking solutions. Endoscope reprocessing consumables, such as detergents, wipes, enzymatic cleaners, and high-level disinfectants, are widely used in routine cleaning and disinfection processes. Equipment, including automated endoscope reprocessors (AERs) and sterilizers, ensures standardized workflows, while tracking solutions provide traceability and compliance support in reprocessing operations.
-
BY TYPE OF ENDOSCOPEBased on type of endoscope, the market is divided into flexible endoscopes and rigid endoscopes. Flexible endoscopes are extensively used in gastrointestinal, pulmonary, and ENT procedures due to their ability to access deeper anatomical structures, whereas rigid endoscopes are commonly applied in surgical interventions requiring precision and stability.
-
BY END USERBy end user, the endoscope reprocessing market is categorized into hospitals & ASCs, specialty clinics, and other end users. Hospitals & ASCs are estimated to account for a major share of endoscopic procedures owing to their advanced infrastructure and higher patient volumes, while specialty clinics and smaller facilities are gradually adopting reprocessing solutions to strengthen infection prevention and control practices.
-
BY REGIONThe Asia Pacific market is estimated to record the highest CAGR between 2025 and 2030, fueled by substantial investments in healthcare infrastructure, a rapidly expanding elderly population, and growing awareness of healthcare-associated infections (HAIs) in countries such as China, India, and those across Southeast Asia.
-
COMPETITIVE LANDSCAPEThe major market players have adopted both organic and inorganic strategies, including acquisitions, partnerships, joint ventures, product launches, expansions, and other developments. Leading companies such as STERIS (Ireland), ASP (US), and Olympus Corporation (Japan) have strengthened their portfolios and expanded their global reach by investing in advanced reprocessing technologies and compliance-driven solutions. These initiatives are aimed at meeting the rising demand for effective infection control, automated disinfection systems, and traceability in endoscope reprocessing across healthcare facilities worldwide.
The global endoscope reprocessing market is witnessing sustained momentum, fueled by the expanding use of endoscopy in the diagnosis and management of chronic and age-related conditions. The rising elderly population, coupled with the growing incidence of gastrointestinal and other disorders, has significantly increased the demand for endoscopic procedures, thereby intensifying the need for reliable reprocessing systems. Healthcare authorities worldwide enforce stringent guidelines to minimize the risk of endoscope-associated infections, further driving market adoption. At the same time, increasing investments in healthcare modernization, infection prevention programs, and technological innovations, particularly in automated and high-level disinfection systems, are shaping the future of the market. These factors collectively ensure continued growth opportunities for manufacturers and solution providers in the years ahead.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The impact on the endoscope reprocessing market is driven by increasing demand for endoscopic procedures and the need to minimize infection risks associated with improperly reprocessed endoscopes. Hospitals, ambulatory surgical centers, and specialized clinics form the core customer base for reprocessing solution providers, with applications spanning gastroenterology, pulmonology, urology, and other minimally invasive procedures. Trends such as the rising endoscope inventory, growing geriatric population, and heightened focus on robust reprocessing protocols and guidelines are reshaping operations and investments in advanced reprocessing equipment and consumables. Despite these favorable dynamics, many manufacturers have yet to fully capitalize on opportunities in high-performance technologies. Over the next three to five years, the expanding adoption of endoscope reprocessing across a broader range of healthcare facilities is expected to significantly influence the market’s growth trajectory and revenue potential.

Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising demand for endoscopy to diagnose and treat target diseases

-
Elevated risk of endoscope-associated infections
Level
-
Persistent concerns about the safety and effectiveness of reprocessed endoscopic devices
-
Potential health hazards associated with exposure to high-level chemical disinfectants
Level
-
Expanding medical devices industry
-
Increasing funding and investments for healthcare infrastructure
Level
-
Preference for single-use endoscopes
-
Increasing number of product failures and recalls
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising demand for endoscopy to diagnose and treat target diseases
The growing prevalence of gastrointestinal diseases, malignancies, and chronic conditions such as GERD, IBD, and pancreatitis has amplified the role of endoscopy as a critical diagnostic and therapeutic tool. With minimally invasive techniques gaining wider acceptance, the adoption of flexible endoscopes has increased, improving both diagnostic accuracy and patient comfort. This rising procedural volume has, in turn, heightened the need for reliable endoscope reprocessing solutions to prevent cross-contamination and ensure patient safety. Moreover, the advancement of specialized procedures such as endoscopic ultrasound and therapeutic colonoscopy has driven healthcare providers to adopt standardized reprocessing protocols in line with safety regulations and best practices.
Restraint: Persistent concerns about safety and effectiveness of reprocessed endoscopic devices
Despite advancements in reprocessing technologies, safety and reliability concerns with reprocessed endoscopes persist. Complex designs, particularly in duodenoscopes, complicate thorough cleaning, increasing contamination and infection risks. Factors such as inconsistent reprocessing protocols, inadequate staff training, and wear of endoscope channels further challenge effective sterilization. Regulatory agencies, including the US FDA and ECDC, have issued multiple safety advisories highlighting these risks. The intricate cleaning required for critical components like biopsy ports, distal tips, and elevator mechanisms necessitates strict oversight and expertise. These ongoing concerns have driven interest in single-use endoscopes, potentially limiting the widespread adoption of conventional reprocessing practices.
Opportunity: Increasing funding and investments for healthcare infrastructure
Governments and healthcare organizations worldwide are investing in modernizing endoscopy suites, improving infection control, and advancing research for safer endoscopic procedures. Funding initiatives are driving innovations in automated reprocessing equipment and essential consumables, supporting the development of advanced endoscopy centers. In emerging markets, international collaborations and public-private partnerships are promoting standardized reprocessing practices, further elevating the quality and safety of gastrointestinal endoscopy care.
Challenge: Preference for single-use endoscopes
The rising use of single-use endoscopes challenges the traditional reprocessing market by reducing cross-contamination risks and lowering operational costs linked to cleaning, storage, and maintenance. Regulatory scrutiny, including FDA advisories on duodenoscope-related infections, has accelerated this shift. Improvements in disposable endoscope technology, such as better optics and ergonomics, have addressed earlier limitations. While initial costs are higher, long-term cost benefits, particularly in outpatient and ambulatory settings, are driving broader adoption, potentially impacting demand for reusable endoscopes and reprocessing systems.
Endoscope Reprocessing Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Provides a comprehensive range of endoscope reprocessing solutions, including AERs, high-level disinfectants, drying and storage cabinets, and integrated tracking software | Enhances patient safety through consistent disinfection, improves workflow efficiency, and ensures full traceability and regulatory compliance |
 |
Offers the AEROFLEX Automatic Endoscope Reprocessor system, which automates endoscope washing and high-level disinfection with fast cycle times and built-in chemical concentration monitoring | Reduces reprocessing time, minimizes manual intervention, and improves safety and consistency in high-level disinfection |
 |
Provides fully integrated reprocessing solutions such as washer-disinfectors (ETD Series), AERs (OER-Mini, OER-Elite), drying cabinets, and Olympus-compatible chemistries | Delivers optimized performance for Olympus endoscopes, ensures reprocessing quality, and increases operational reliability |
 |
Offers endoscope washer-disinfectors and advanced drying/storage cabinets (EDS8), supported by T-DOC software for digital traceability and documentation | Improves infection control, extends the microbiological safety window of stored scopes, and facilitates compliance with reprocessing standards |
 |
Provides Soluscope-branded automated washer-disinfectors, enzymatic detergents, high-level disinfectants, drying cabinets, and endoscope transport solutions | Ensures effective cleaning and disinfection, supports fast turnaround, and helps maintain compliance with regulatory and safety standards |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The endoscope reprocessing market ecosystem comprises a network of manufacturers, distributors, healthcare facilities, and development partners collaborating to ensure safe and efficient endoscope use. Manufacturers drive innovation by developing advanced reprocessing equipment, consumables, and tracking systems, often supported by contract research organizations (CROs) and specialized product developers. Distributors, logistics providers, and group purchasing organizations ensure timely availability across regional and global markets. Hospitals, ambulatory surgical centers, and specialty clinics serve as the primary end-users, implementing reprocessing solutions to comply with infection control standards and minimize cross-contamination risks. Strategic partnerships, technological advancements, and investments are expanding access to high-performance reprocessing solutions, enabling broader adoption and enhancing patient safety across healthcare settings.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS

Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Endoscope Reprocessing Market, By Product
By product, endoscope reprocessing consumables held the dominant share of the endoscope reprocessing market in 2024. These include detergents, wipes, enzymatic cleaners, and high-level disinfectants, which are repeatedly required in cleaning and disinfection cycles. Their frequent utilization, combined with healthcare facilities’ strict adherence to infection control protocols, drives consistent demand. The growing emphasis on compliance, traceability, and the use of single-use consumables has further strengthened the position of this segment. Equipment and tracking solutions also play an important role, but consumables continue to generate the largest recurring revenue stream.
Endoscope Reprocessing Market, By Type of Endoscope
Flexible endoscopes accounted for the largest market share in 2024. Their widespread adoption in gastrointestinal, pulmonary, and ENT procedures highlights their importance in minimally invasive diagnostics and treatments. The complexity of flexible endoscope reprocessing necessitates specialized automated endoscope reprocessors (AERs) and dedicated cleaning agents, fueling innovation and adoption of advanced reprocessing systems. In comparison, rigid endoscopes have more limited applications, contributing to a smaller market share.
Endoscope Reprocessing Market, By End User
Hospitals and ASCs dominated the market in 2024. This dominance is attributed to the high patient throughput in these facilities, robust infrastructure for reprocessing, and strict compliance with infection prevention standards. The increasing burden of gastrointestinal, respiratory, and urological disorders, especially among aging populations, has led to higher procedure volumes, driving greater utilization of reprocessing products. The presence of dedicated sterile processing departments and the integration of advanced tracking systems further strengthen the role of hospitals and ASCs as the primary end users.
REGION
Asia Pacific to be fastest-growing region in global endoscope reprocessing market during forecast period
The Asia Pacific endoscope reprocessing market is expected to record the highest CAGR from 2025 to 2030, driven by rising healthcare investments, a growing aging population, and increased awareness of healthcare-associated infections (HAIs) in China, India, and Southeast Asia. Growth is further supported by favorable government initiatives, the expansion of private hospital networks, and regional localization strategies by key manufacturers. In 2024, China accounted for the largest share of the region’s market, fueled by its large patient base, rising prevalence of chronic and gastrointestinal diseases, and modernization of hospital sterilization departments. Government efforts to improve healthcare quality, coupled with the rapid expansion of endoscopy services, have significantly heightened demand in the country.

Endoscope Reprocessing Market: COMPANY EVALUATION MATRIX
In the endoscope reprocessing market matrix, STERIS (Star) leads with a strong global presence and a comprehensive portfolio of automated endoscope reprocessors (AERs), consumables, and tracking solutions, driving widespread adoption across hospitals, ASCs, and specialty clinics. Fujifilm Corporation (Emerging Leader) is gaining momentum with innovative reprocessing systems and technologies that enhance workflow efficiency and compliance with infection control standards, positioning the company for rapid growth. While STERIS dominates with scale, innovation, and established market leadership, Fujifilm demonstrates strong potential to advance toward the leaders’ quadrant.

Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size, 2024 (Value) | USD 2.49 Billion |
| Market Forecast, 2030 (Value) | USD 4.24 Billion |
| Growth Rate | CAGR of 9.4% from 2025 to 2030 |
| Study Period | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
|
| Regional Scope | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
WHAT IS IN IT FOR YOU: Endoscope Reprocessing Market REPORT CONTENT GUIDE

RECENT DEVELOPMENTS
- May 2025 : Olympus launched the ScopeLocker Air endoscope drying cabinet, designed to improve infection prevention by ensuring optimal drying and storage of endoscopes.
- December 2024 : HOYA Corporation acquired the remaining shares of PLASMABIOTICS, becoming the sole owner of the France-based medical reprocessing company known for its PlasmaTYPHOON and AquaTYPHOON systems that enhance the reprocessing cycle for flexible endoscopes.
- September 2024 : Olympus Australia opened Sapphire, its first flexible endoscope sterilization facility in Melbourne. As part of the Olympus On-Demand solution, the facility delivers sterile, ready-to-use flexible endoscopes directly to hospitals, reducing risks, costs, and complexity in endoscopy services.
- June 2024 : Metall Zug (33%) and Miele/Steelco (67%) formed SteelcoBelimed, a joint venture merging Steelco and Belimed’s hospital infection-control and life-science equipment operations in Zug, Switzerland.
- June 2022 : Getinge introduced Vac-a-Scope, a patented benchtop packaging system that prepares and stores endoscopes, maintaining instrument integrity from reprocessing to procedure room, thereby preventing healthcare-associated infections (HCAIs).
Table of Contents

Methodology
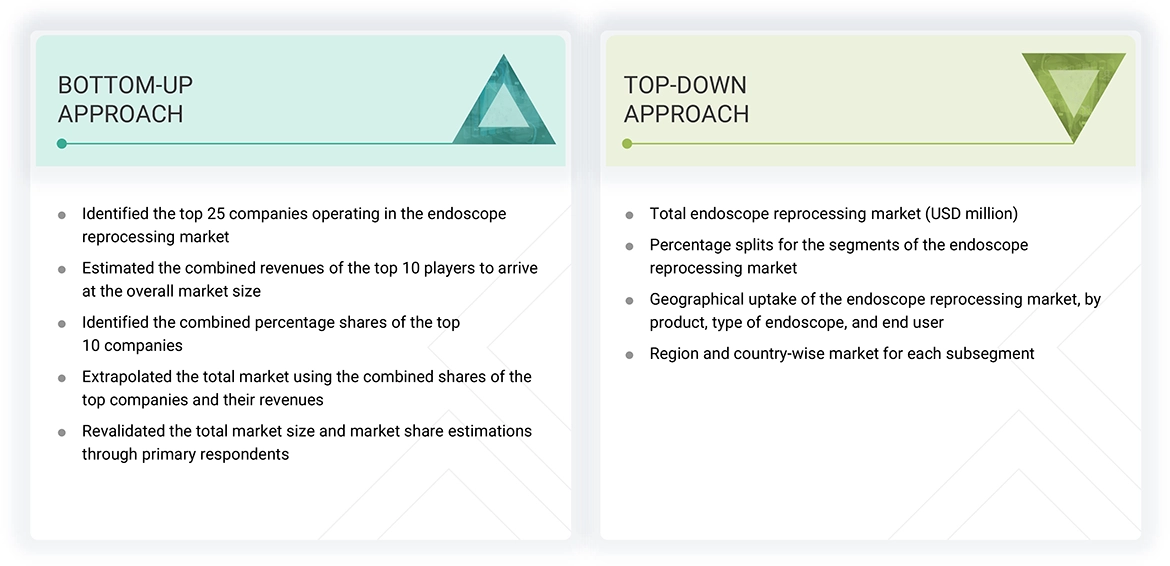
The study involved key activities in estimating the current market size for the endoscope reprocessing market. Extensive secondary research was conducted to gather information on this sector. The next step was to validate these findings, assumptions, and size estimates with industry experts across the value chain through primary research. Different approaches, such as top-down and bottom-up methods, were used to calculate the total market size. Subsequently, market segmentation and data triangulation techniques were applied to estimate the size of various segments and subsegments within the endoscope reprocessing market.
The four steps involved in estimating the market size are:
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, investor presentations, SEC filings of companies and publications from government sources [such as National Institutes of Health (NIH), US FDA, US Census Bureau, World Health Organization (WHO), , Global Burden of Disease Study, and Centers for Medicare and Medicaid Services (CMS) were referred to identify and collect information for the global endoscope reprocessing market study. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends, to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
During the primary research process for the endoscope reprocessing market, various stakeholders from both supply and demand sides were interviewed to gather qualitative and quantitative insights. On the supply side, primary sources included industry experts such as CEOs, vice presidents, marketing and sales directors, product development managers, and heads of technology and innovation from leading companies offering endoscope reprocessing equipment, consumables, and tracking systems. On the demand side, respondents included infection control specialists, hospital procurement officers, sterilization technicians, and representatives from ambulatory surgical centers (ASCs), specialty clinics, and hospitals. The primary research aimed to validate the segmentation framework, identify key players and their strategies, and understand current and emerging trends, challenges, and market dynamics impacting the endoscope reprocessing industry.
The following is a breakdown of the primary respondents:

Note 1: Other designations include sales managers, marketing managers, and product managers.
Note 2: Companies are classified into tiers based on their total revenues. As of 2024, Tier 1 = >USD 100 million, Tier 2 = USD 10 million to USD 100 million, and Tier 3 = < USD 10 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
For the global market value, annual revenues were determined based on revenue mapping of leading product manufacturers and OEMs active in the worldwide endoscope reprocessing market. All key service providers were identified at the global, national, and/or national/regional levels. Revenue mapping for the respective business segments and sub-segments was conducted for the major players. The global endoscope reprocessing market was split into various segments and subsegments based on:
- List of major players operating in the products market at the regional and/or country level
- Product mapping of endoscope reprocessing providers at the regional and/or country level
- Mapping of annual revenue generated by listed major players from endoscope reprocessing (or the nearest reported business unit/product category)
- Extrapolation of the revenue mapping of the listed major players to derive the global market value of the respective segments/subsegments
- Summation of the market value of all segments/subsegments to arrive at the global endoscope reprocessing market
The above-mentioned data was consolidated and added with detailed inputs and analysis from MarketsandMarkets and presented in this report.
The research methodology used to estimate the market size includes the following:
Data Triangulation
After determining the overall market size using the process outlined above, the total market was divided into several segments. To complete the market engineering process and obtain precise data for all segments and subsegments, data triangulation and market breakdown methods were used wherever applicable. The data was triangulated by examining various factors and trends from both the demand and supply sides.
Market Definition
Endoscope reprocessing involves cleaning, high-level disinfection, or sterilization of reusable endoscopes to ensure safe reuse and prevent cross-contamination. The market includes automated reprocessing equipment (such as automated endoscope reprocessors (AER) and endoscope drying, storage, and transport systems), consumables (such as detergents, wipes, and disinfectants), and tracking systems that help maintain compliance with infection control standards. These solutions are essential for reducing healthcare-associated infections (HAIs) and are commonly used across hospitals, ambulatory surgical centers, and specialty settings clinics.
Stakeholders
- Endoscope Reprocessing Equipment and Consumables Manufacturers
- Hospitals and Ambulatory Surgical Centers (ASCs)
- Endoscopy Units and Clinics
- Distributors and Suppliers of Endoscope Reprocessing Solutions
- Contract Manufacturers of Endoscope Reprocessing Devices
- Research & Consulting Firms Focused on Infection Control and Reprocessing
- Regulatory Bodies and Government Health Organizations
- Venture Capitalists and Investors
- Government Organizations
Report Objectives
- To define, describe, segment, and forecast the endoscope reprocessing market by product, type of endoscope, end user, and region
- To provide detailed information regarding the major factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall endoscope reprocessing market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of the market segments with respect to five regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To benchmark players within the market using the proprietary Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business and product excellence
- To study the impact of AI/Gen AI on the market, along with the macroeconomic outlook for each region
Key Questions Addressed by the Report
Which are the top industry players in the global endoscope reprocessing market?
The top market players in the global endoscope reprocessing market include ASP (US), Olympus Corporation (Japan), Ecolab (US), STERIS (Ireland), and Getinge AB (Sweden).
What are some of the major drivers for this market?
Rising geriatric population and growing adoption of minimally invasive surgeries are expected to drive the market.
Which end users have been included in the global endoscope reprocessing market?
This report contains the following end-user segments: hospitals and ambulatory surgery centers, specialty clinics, and other end users.
Which type of endoscope is expected to register the highest growth rate in the market?
The flexible endoscopes segment is expected to witness the highest growth rate during the forecast period.
Which region is lucrative in the endoscope reprocessing market?
The Asia Pacific market is expected to witness the highest CAGR during the forecast period.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Endoscope Reprocessing Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Endoscope Reprocessing Market