Cystatin C Assay Market by Product (Analyzers, Kits, Reagents), Method (ELISA, PETIA, IFA, CLIA, PENIA), Application (Diagnostics, Research), Sample Type (Blood, Urine), End User (Hospitals, Clinical Laboratories) & Region - Global Forecast to 2028
Cystatin C Assay Market Size, Growth Drivers & Restraints
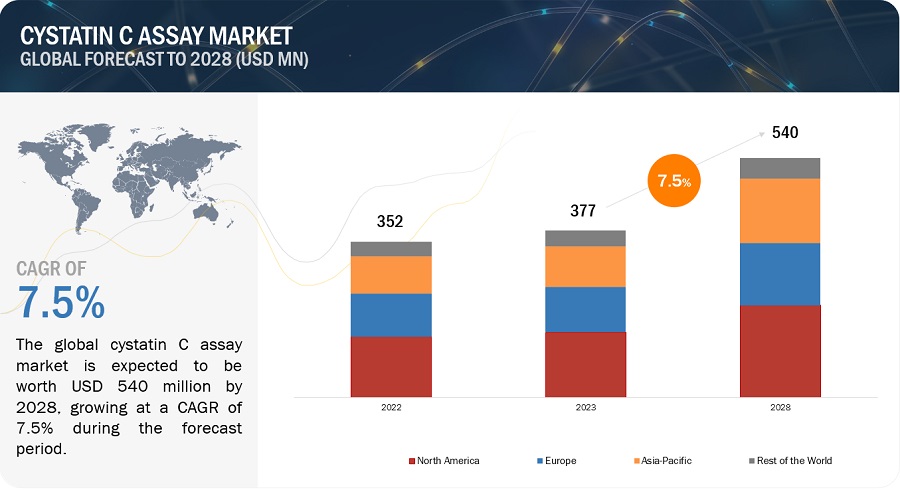
The global cystatin C assay market, valued at US$352 million in 2022, stood at US$377 million in 2023 and is projected to advance at a resilient CAGR of 7.5% from 2023 to 2028, culminating in a forecasted valuation of US$540 million by the end of the period. The growth of this market is majorly driven by the Increasing adoption of POC testing and presence of supportive government policies. However, raising awareness among healthcare providers about the benefits of cystatin C testing can be challenging.
Attractive Opportunities in the Cystatin C Assay Market
To know about the assumptions considered for the study, Request for Free Sample Report
Recession Impact on the Cystatin C Assay Market
During a recession, governments may face budget constraints and prioritize spending on essential services. This can lead to potential reductions in healthcare budgets, affecting reimbursement rates and procurement decisions for Cystatin C Assay products. Likewise, healthcare providers and institutions may face financial constraints and look for ways to reduce costs. This can lead to increased price sensitivity and potential pricing pressure on bone cement and glue products.
On the other hand, the conflict between Ukraine and Russia has the potential to create economic challenges and disrupt regional stability. It is possible that the conflict could contribute to a recession in the affected regions and have broader economic implications. The conflict can disrupt supply chains, leading to challenges in the procurement and distribution of bone cement and glue products. Transport routes may be affected, making it difficult to deliver products to healthcare facilities in conflict-affected areas. During economic downturns, healthcare budgets may be constrained, leading to reduced spending on diagnostic tests, including Cystatin C testing. Hospitals and healthcare facilities may prioritize essential services over non-urgent tests.
Cystatin C Assay Market Dynamics
Driver: Rising Prevalence of Kidney Diseases
Kidney diseases and disorders, including chronic kidney disease (CKD), are becoming more prevalent globally due to factors such as aging populations, hypertension, diabetes, and lifestyle changes. Cystatin C assays are valuable tools for accurately assessing kidney function and diagnosing kidney-related issues.
Chronic kidney disease (CKD) is becoming one of the world's most prevalent noncommunicable chronic diseases. The World Health Organization projects CKD to become the 5th most common chronic disease in 2040. (Source: PubMed 2023). Similarly, it is a progressive condition that affects >10% of the general population worldwide, amounting to >800 million individuals (Source: PubMed 2022). In addition, more than 850 million individuals have kidney diseases worldwide. (Source: AstraZeneca 2023).
As per the Pan American Health Organization 2021. In 2019, regionwide kidney diseases account for, 254,028 total deaths, 131,008 deaths in men, and 123,020 deaths in women. The age-standardized death rate due to kidney diseases was estimated at 15.6 deaths per 100,000 population. The countries with the highest age-standardized death rates due to kidney diseases are Nicaragua, El Salvador, Bolivia, Guatemala, Suriname, Honduras, and Ecuador.
More than 1 in 7 US adults–about 35.5 million people, or 14%–are estimated to have CKD (Source: CDC: 2023)
Likewise, Diabetes is a major risk factor for kidney diseases, particularly CKD. As the global incidence of diabetes continues to rise due to factors such as sedentary lifestyles and poor dietary habits, the prevalence of kidney diseases linked to diabetes also increases. For instance, as per the Institute of Health Metrics and Evaluation at the University of Washington found that in 2022, there were 529 million people in the world with diabetes. They projected that this will more than double to around 1.3 billion people by 2050. As
Similarly, rising government as well as private funding for kidney diseases drive the market. Government funding plays a crucial role in advancing research, diagnosis, and treatment of kidney diseases, and this funding can have a direct and positive impact on the market.
For instance, in 2023, the National Kidney Foundation (NKF) announced its latest investment through the NKF's Innovation Fund in Klinrisk, an artificial intelligence-based medical device company. Klinrisk is dedicated to transforming the early identification and management of high-risk chronic kidney disease (CKD) through accurate clinical risk prediction and decision support.
In 2023, Healthy.io, the global leader in transforming the smartphone camera into a clinical-grade device, announced that it completed a USD 50 million investment. This investment, together with a previously unannounced USD 45 million February 2022 investment, comprise the company's Series D. The company has seen increased commercial traction in the United States since July 2022, when it became the first and only company to offer an FDA-cleared smartphone-powered, at-home kidney test. Likewise, this Series D funding round is led by Schusterman Family Investments and is joined by Aleph and other existing shareholders. This investment, together with a previously unannounced USD 45 million February 2022 investment, comprise the company's Series D. The company has seen increased commercial traction in the United States since July 2022, when it became the first and only company to offer an FDA-cleared smartphone-powered, at-home kidney test.
In 2021, Monogram Health, a benefit management and care delivery company transforming care for individuals with chronic kidney and end-stage renal disease, announced that it closed a USD 160 million Series B funding round led by TPG Capital, the private equity platform of global alternative asset firm TPG.
In 2020, The Australian Government invested almost USD 35 million in Indigenous health projects including Indigenous blindness, deafness and chronic kidney disease.
Cystatin C assays offer the capability of detecting kidney dysfunction at an earlier stage, which is crucial for implementing interventions and treatments to slow down or manage the progression of kidney diseases. Additionally, cystatin C testing provides a means for monitoring kidney function over time. A result of the rising incidences of kidney diseases across the globe is expected to drive the demand of Cystatin assay market.
Restraint: High Development Costs of cystatin C assay
The cost of research, development, and validation of cystatin C assays can be a barrier for smaller diagnostic companies. Moreover, the costs associated with developing, producing, and implementing cystatin C assays, along with the required equipment and training, can pose a financial challenge for some healthcare facilities, especially in resource-constrained settings. For instance, the cost, accessibility, and the level of clinical awareness and understanding of test results. Cystatin C testing is approximately ten times more costly than creatinine testing, with a price of GBP 2.50 (USD 3.00) per test compared to GBP 0.25 (USD 0.30) for creatinine (Source: PubMed 2023). Likewise, CysC assays can run on standard analyzers housed in most laboratories, CysC and sCr testing have similar labor costs. However, CysC reagents cost around USD 5–USD 10 per test compared with around USD 0.50 per test for sCr within the US; this cost differential will likely decline as CysC testing increases. In cost simulations of implementing CysC testing using the Gentian assay in Canada, the cost was estimated to be USD 2.88 per test for 1200 tests per year versus USD 2.17 per test based on 10,000 tests per year. Similarly, a cystatin C test may currently cost more than a serum creatinine assay, the price of the former is not prohibitive. At Karger's institution, the University of Minnesota, a serum creatinine test costs about USD 2.50, while a cystatin C assay runs about USD 10.60 (Source: Medscape 2021).
Opportunity: Importance of companion diagnostics
Companion diagnostics include tests or assays intended to assist healthcare providers in making treatment decisions for patients based on the best response to therapy. The co-development of companion diagnostics with therapeutic products can significantly alter the drug development process and commercialize drug candidates by yielding safer drugs with enhanced therapeutic efficacy quickly and cost-effectively. With an increase in the demand for high-priced specialist therapies and safer drugs, the market for companion diagnostics is expected to showcase a high growth potential. The growing importance of companion diagnostics provides growth opportunities for the overall diagnostics market and the market.
Cystatin C, a protein that plays a role in inhibiting certain enzymes, has garnered interest in cancer research due to its potential implications in various aspects of cancer development, progression, and treatment response. In addition, pharmaceutical companies are increasingly collaborating with diagnostic companies to make safer and more effective drugs. On July 8, 2020, Thermo Fisher Scientific, Inc. (US) signed a Companion Diagnostic Agreement with Chugai Pharmaceutical Co., Ltd., (Japan), a Roche Group member. The company has also applied to the Ministry of Health, Labour and Welfare (MHLW) to elevate the use of the Oncomine Dx Target assay in Japan. Similarly, Cystatin C assay has gained importance in companion diagnostics, particularly in the field of nephrology and kidney-related diseases. Cystatin C is a sensitive and reliable marker of kidney function. It is particularly useful for the early detection and monitoring of kidney diseases, including chronic kidney disease (CKD). Companion diagnostics that include Cystatin C assays can help identify kidney dysfunction at an earlier stage, allowing for prompt intervention and management
Challenge: Dearth of skilled professionals
The scarcity of skilled professionals in the market is indeed a challenge that can affect the effective implementation and adoption of this diagnostic tool. The field of diagnostic testing requires trained personnel who can perform assays accurately, interpret results, troubleshoot technical issues, and ensure quality control. The dearth of a skilled workforce has been a challenge for several decades, resulting in an aging workforce and declining enrolment in training programs. It takes almost five to ten years of continuous practice in clinical laboratory work for technicians to gain expertise.
Europe and the UK have already reported low numbers of clinical technicians. The Gatsby Foundation stated in 2019 that over 1.5 million people serve in the health, engineering, science, and technology domains. Over 50,000 retire every year, and 700,000 technicians will be required to meet the soaring demands in the next decade. The Duquesne University School of Nursing has stated that by 2025, there will be a shortage of over 29,400 nurse practitioners and more than 400,000 home health aides
Ecosystem Analysis of Cystatin C Assay Market
An ecosystem analysis of the cystatin C assay would involve examining the various stakeholders, components, and interactions within the ecosystem that contribute to the development, adoption, and utilization of cystatin C assays in clinical and research settings. Moreover, an ecosystem analysis of the cystatin C assay helps stakeholders understand the dynamics, challenges, and opportunities within this healthcare sector. Collaboration among stakeholders and ongoing efforts to address challenges can contribute to the advancement of cystatin C testing and its impact on patient care and research.
Based on product, the cystatin C assay market is segmented into analyzers, kits, and reagents. The reagents segment is expected to account the second largest shares during the forecast period. The demand for separate reagents is driven by the specific needs of clinical laboratories and research institutions. Separate reagents are also used in clinical laboratories and research settings. The availability of high-quality reagents is crucial for accurate and consistent cystatin C measurements, ultimately contributing to the overall growth of the market.
Based on method, the cystatin C assay market is segmented into Enzyme-Linked Immunosorbent Assay (ELISA), Particle-Enhanced Turbidimetric Immunoassay (PETIA), Particle-enhanced Nephelometric Immunoassay (PENIA), Chemiluminescent immunoassay (CLIA), Immunofluorescence assay (IFA), and Others. The PETIA segment accounted for the second largest share the market in 2022 due its advantages such as many clinical laboratories prefer automated methods like PETIA because they can be easily integrated into clinical chemistry analyzers, offering efficiency and reducing the potential for manual errors. The demand for cystatin C assay methods, as PETIA, continues to be driven by the need for precise and reliable measurements in the diagnosis and monitoring of kidney function, particularly in the context of chronic kidney disease (CKD) and related conditions.
Market Segments of Cystatin C Assay Market
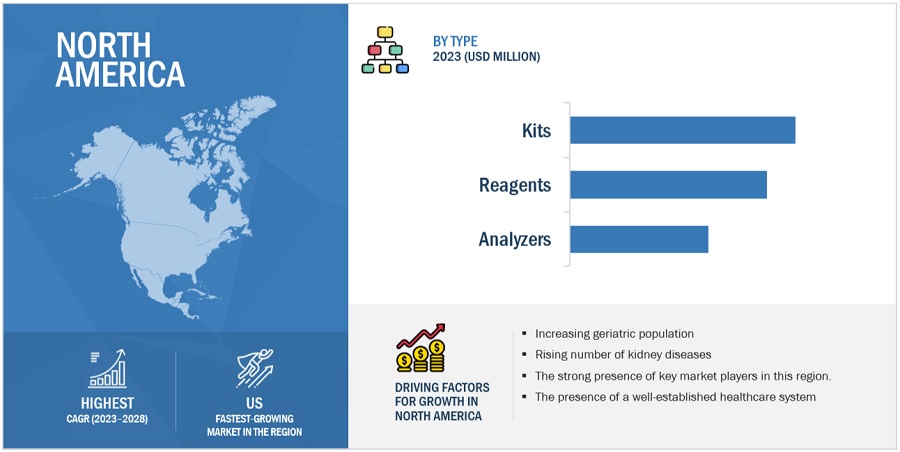
US accounted for the largest share of the North American cystatin c assay industry in 2022.
Based on the region, the North American cystatin c assay market is segmented into US and Canada. In 2022, the US accounted for the largest share of the North American market. Growth in this market can be attributed to presence of stringent regulatory standard, rising healthcare expenditure, the increasing number of chronic kidney diseases, the rising geriatric population, and the strong presence of cystatin c assay manufacturers.
To know about the assumptions considered for the study, download the pdf brochure
India registered the second highest growth rate in Asia Pacific cystatin C assay industry.
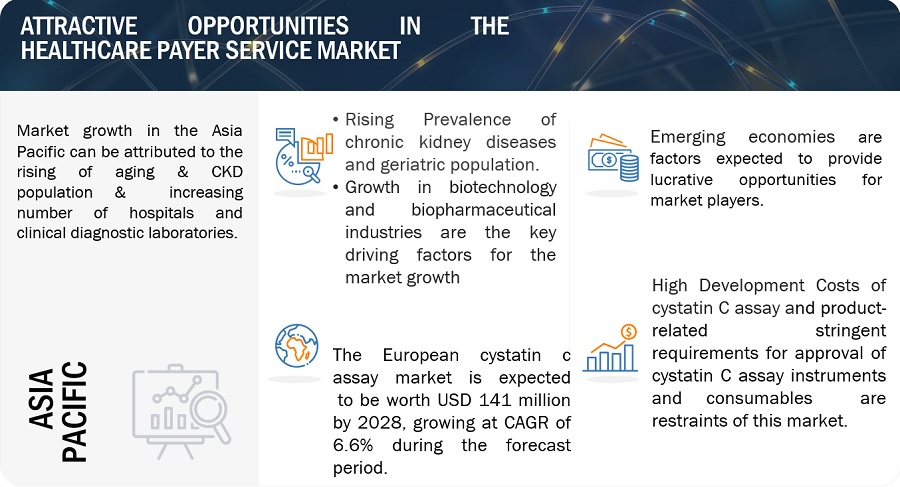
The APAC cystatin C assay market is segmented into Japan, China, India, and Rest of Apac. In 2022, India accounted for the second highest growth rate of the Asian market. The second high growth rate of India can be attributed to the increasing awareness of health issues, and rising healthcare expenditure, rising of a medical tourism and the increasing geriatric population. An increase in the number of elderly people gives rise to various nephros diseases. Therefore, growth in this population segment will directly drive the demand for cystatin C assay products.
The France in European cystatin C assay industry to witness the second highest shares during the forecast period.
The Europe cystatin C assay market is segmented into Germany, France, the UK, Italy, Spain and the Rest of Europe. France is registered the second highest shares during the forecast period. The major factors contributing to the growth of this market are the high prevalence of old age population, rising in kindey diseases, increasing R&D activities, strategic developments by key players. Clinical laboratories and diagnostic centers are key users of cystatin C assays in France. These facilities perform a wide range of diagnostic tests, including kidney function assessments, contributing to the demand for cystatin C assays.
Some of the major players operating in this market are Abbott (US), Roche Diagnostics Limited.(Switzerland), Siemens Healthcare GmbH (Germany),Thermo Fisher Scientific Inc. (US), Randox Laboratories Ltd. (UK), DiaSys Diagnostic Systems GmbH (Germany), Bio-Techne (US), Gentian Diagnostics ASA (Norway), Getein Biotech, Inc. (China), Agilent Technologies, Inc. (US), Abcam plc. (UK), Sino Biological, Inc. (China), Eurolyser Diagnostica GmbH (Austria).
In 2022, Roche Diagnostics Limited. (Switzerland), held the leading position in the market. The company’s large share can be attributed to broad usage of its analyzers and strong global presence. Abbott (US), held the second position in the cystatin c assay market in 2022.
Scope of the Cystatin C Assay Industry:
|
Report Metric |
Details |
|
Market Revenue in 2023 |
$377 million |
|
Estimated Value by 2028 |
$540 million |
|
Revenue Rate |
Poised to grow at a CAGR of 7.5% |
|
Market Driver |
Rising Prevalence of Kidney Diseases |
|
Market Opportunity |
Importance of companion diagnostics |
This research report categorizes the cystatin C assay market to forecast revenue and analyze trends in each of the following submarkets:
|
SEGMENTS |
INCLUSIONS |
|
Product |
|
|
Method |
|
|
Sample Type |
|
|
Application |
|
|
End User |
|
|
Region |
|
Recent Developments of Cystatin C Assay Industry
- September 2022, Gentian Cystatin C and GCAL assays received IVDR (In-Vitro Diagnostic Regulation) by TüV SÜD.
- March 2021, Roche acquired GenMark Diagnostics at USD 24.05 per share and a total transactional value of USD 1.8 billion. The acquisition strengthened Roche’s Diagnostics portfolio.
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the global cystatin C assay market?
The global cystatin C assay market boasts a total revenue value of $540 million by 2028.
What is the estimated growth rate (CAGR) of the global cystatin C assay market?
The global cystatin C assay market has an estimated compound annual growth rate (CAGR) of 7.5% and a revenue size in the region of $377 million in 2023.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst


- 5.1 INTRODUCTION
-
5.2 MARKET DYNAMICSDRIVERS- Rising prevalence of kidney diseases- Growing geriatric population- Recent advancements in chemiluminescence immunoassay technologies- Growth in biotechnology and biopharmaceutical industries- Increasing adoption of POC testing- Supportive government policiesRESTRAINTS- Stringent requirements for approval of cystatin C assay instruments and consumables- High development costs of cystatin C assaysOPPORTUNITIES- Growth opportunities in emerging economies- Importance of companion diagnostics- Development of condition-specific biomarkers and testsCHALLENGES- Dearth of skilled professionals
-
5.3 REGULATORY OVERVIEWUSCANADAEUROPEJAPAN- Japan: Regulatory process for IVD devicesCHINAINDIA- India: Regulatory process for IVD devicesINDONESIARUSSIASAUDI ARABIAMEXICO- Mexico: Regulatory process for IVD devicesBRAZIL- Brazil: Regulatory process for IVD devicesSOUTH KOREAMIDDLE EASTAFRICA
- 5.4 TECHNOLOGY ANALYSIS
-
5.5 TRADE ANALYSISTRADE ANALYSIS FOR IMMUNOASSAYS
-
5.6 PATENT ANALYSIS
- 5.7 VALUE CHAIN ANALYSIS
- 5.8 SUPPLY CHAIN ANALYSIS
-
5.9 ECOSYSTEM ANALYSIS OF CYSTATIN C ASSAY MARKETROLE OF COMPANIES IN ECOSYSTEMKEY PLAYERS OPERATING IN CYSTATIN C ASSAY MARKET
-
5.10 PORTER’S FIVE FORCES ANALYSISINTENSITY OF COMPETITIVE RIVALRYBARGAINING POWER OF SUPPLIERSBARGAINING POWER OF BUYERSTHREAT FROM SUBSTITUTESTHREAT FROM NEW ENTRANTS
- 5.11 KEY CONFERENCES & EVENTS (2023-2024)
- 5.12 PRICING ANALYSIS
-
5.13 KEY STAKEHOLDERS & BUYING CRITERIAKEY STAKEHOLDERS IN BUYING PROCESSBUYING CRITERIA
-
5.14 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 6.1 INTRODUCTION
-
6.2 KITSWIDE USE OF KITS IN CLINICAL LABORATORIES, HEALTHCARE SETTINGS, AND RESEARCH INSTITUTIONS TO DRIVE MARKETREAGENTS- Wide use in diagnostic, research, and high-throughput screening to drive market
-
6.3 ANALYZERSINCREASING NUMBER OF HOSPITALS AND CLINICAL LABORATORIES TO DRIVE MARKET
- 7.1 INTRODUCTION
-
7.2 ENZYME-LINKED IMMUNOSORBENT ASSAYWIDE ROLE IN DIAGNOSIS, PROGNOSIS, AND MONITORING OF VARIOUS RENAL DISEASES TO DRIVE MARKET
-
7.3 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAYAPPLICATION OF PETIA TECHNOLOGY IN ALL CLINICAL ANALYZERS TO DRIVE MARKET
-
7.4 IMMUNOFLUORESCENCE ASSAYWIDE USE OF IMMUNOFLUORESCENCE ASSAY IN DIAGNOSIS OF ANTIBODIES IMMUNOLOGY AND CELL BIOLOGY RESEARCH TO DRIVE MARKET
-
7.5 CHEMILUMINESCENT IMMUNOASSAYACCESSIBILITY TO MANY CLINICAL LABORATORIES TO INCREASE DEMAND
-
7.6 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAYPRECISION AND AUTOMATION CAPABILITIES IN CLINICAL LABORATORIES TO DRIVE MARKET
- 7.7 OTHER METHODS
- 8.1 INTRODUCTION
-
8.2 BLOODRISING PREVALENCE OF CHRONIC CONDITIONS TO DRIVE MARKET
-
8.3 URINERESEARCH APPLICATION OF URINE CYSTATIN C IN CLINICAL INVESTIGATION TO DRIVE MARKET
- 9.1 INTRODUCTION
-
9.2 DIAGNOSTICSWIDE USE OF CYSTATIN C ASSAYS IN DIAGNOSTICS TO DRIVE MARKET
-
9.3 RESEARCHUSE OF CYSTATIN C ASSAYS IN SCIENTIFIC STUDIES, CLINICAL TRIALS, AND BIOMARKER RESEARCH TO BOOST MARKET
- 10.1 INTRODUCTION
-
10.2 HOSPITALSGROWING PATIENT POPULATION TO DRIVE MARKET
-
10.3 CLINICAL LABORATORIESRISING CLINICAL TEST VOLUMES AND REQUIREMENTS TO BOOST USE OF CYSTATIN C ASSAYS
-
10.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CONTRACT RESEARCH ORGANIZATIONS, AND ACADEMIC RESEARCH INSTITUTESGROWING DRUG DISCOVERY AND CLINICAL STUDY AND RISING NUMBER OF COLLEGES & UNIVERSITIES TO DRIVE DEMAND
- 11.1 INTRODUCTION
-
11.2 NORTH AMERICARECESSION IMPACT ON NORTH AMERICAUS- US to account for largest market share in North AmericaCANADA- Government initiatives to support market growth
-
11.3 EUROPERECESSION IMPACT ON EUROPEGERMANY- High healthcare spending to drive marketFRANCE- Presence of favorable reimbursement policies to make market lucrativeUK- Government support for disease diagnostics and favorable investment scenario to drive marketITALY- Growing geriatric population and increasing support for research to boost market growthSPAIN- Presence of a well-established network of research centers, universities, and hospitals to drive marketREST OF EUROPE
-
11.4 ASIA PACIFICRECESSION IMPACT ON ASIA PACIFICCHINA- High prevalence of diabetes and chronic kidney disease to drive marketINDIA- Growing medical tourism and healthcare infrastructure to drive marketJAPAN- Investments in healthcare technology and research to support growthREST OF ASIA PACIFIC
-
11.5 REST OF THE WORLDRECESSION IMPACT ON REST OF THE WORLD

- 12.1 OVERVIEW
- 12.2 STRATEGIES ADOPTED BY KEY PLAYERS
- 12.3 MARKET RANKING ANALYSIS, 2022
- 12.4 REVENUE SHARE ANALYSIS, 2022
-
12.5 COMPANY EVALUATION MATRIXSTARSPERVASIVE PLAYERSPARTICIPANTSEMERGING LEADERS
-
12.6 SMES/STARTUPS EVALUATION MATRIXPROGRESSIVE COMPANIESSTARTING BLOCKSRESPONSIVE COMPANIESDYNAMIC COMPANIES
-
12.7 COMPETITIVE BENCHMARKINGCOMPANY FOOTPRINT ANALYSIS
-
12.8 COMPETITIVE SCENARIOPRODUCT LAUNCHES AND APPROVALSDEALS
-
13.1 KEY PLAYERSROCHE DIAGNOSTICS LIMITED- Business overview- Products offered- Recent developments- MnM viewABBOTT- Business overview- Products offered- MnM viewTHERMO FISHER SCIENTIFIC INC.- Business overview- Products offered.- Recent developments- MnM viewSIEMENS HEALTHCARE GMBH- Business overview- Products offered.BIO-TECHNE- Business overview- Products offered.AGILENT TECHNOLOGIES, INC.- Business overview- Products offeredGENTIAN DIAGNOSTICS ASA- Business overview- Products offered- Recent developments- MnM viewABCAM PLC- Business overview- Products offered.- Recent developmentsGETEIN BIOTECH, INC.- Business overview- Products offeredSINO BIOLOGICAL, INC.- Business overview- Products offered.EUROLYSER DIAGNOSTICA GMBH- Business overview- Products offeredDIAZYME LABORATORIES, INC- Business overview- Products offeredKAMIYA BIOMEDICAL COMPANY- Business overview- Products offeredRANDOX LABORATORIES LTD.- Business overview- Products offeredDIASYS DIAGNOSTIC SYSTEMS GMBH- Business overview- Products offeredCEPHAM LIFE SCIENCES- Business overview- Products offered
-
13.2 OTHER PLAYERSETHOS BIOSCIENCESIMMUNODIAGNOSTICS LIMITEDSEKISUI DIAGNOSTICSAALTO SCIENTIFIC, LTDRAYBIOTECH LIFE, INC.ARBOR ASSAYSCUSABIO TECHNOLOGY LLCPROTEINTECH GROUP, INC.ZHEJIANG KANGTE BIOTECHNOLOGY CO., LTD.
- 14.1 INSIGHTS FROM INDUSTRY EXPERTS
- 14.2 DISCUSSION GUIDE
- 14.3 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
- 14.4 AVAILABLE CUSTOMIZATIONS
- 14.5 RELATED REPORTS
- 14.6 AUTHOR DETAILS
- TABLE 1 RISK ASSESSMENT: CYSTATIN C ASSAY MARKET
- TABLE 2 GERIATRIC POPULATION, BY REGION, 2015 VS. 2030 VS. 2050 (MILLION)
- TABLE 3 ESTIMATED INCREASE IN GERIATRIC POPULATION, BY REGION (2019–2050)
- TABLE 4 POC ANALYSIS DEVICES
- TABLE 5 CYSTATIN C CONSUMABLES AND INSTRUMENTS
- TABLE 6 APPROVED AND LAUNCHED COMPANION DIAGNOSTIC ASSAYS
- TABLE 7 EUROPE: CLASSIFICATION OF IVD DEVICES
- TABLE 8 JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 9 CHINA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 10 INDONESIA: REGISTRATION PROCESS FOR IVD DEVICES
- TABLE 11 RUSSIA: CLASSIFICATION OF IVD DEVICES
- TABLE 12 SAUDI ARABIA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 13 MEXICO: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 14 SOUTH KOREA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
- TABLE 15 NON-GFR DETERMINANTS OF SERUM CREATININE AND CYSTATIN C
- TABLE 16 RECENT PRODUCT LAUNCHES WITH ADVANCED TECHNOLOGIES IN CYSTATIN C ASSAY MARKET
- TABLE 17 IMPORT DATA FOR HS CODE 902750, BY COUNTRY, 2018–2022 (USD THOUSAND)
- TABLE 18 EXPORT DATA FOR HS CODE 902750, BY COUNTRY, 2018–2022 (USD THOUSAND)
- TABLE 19 IMPACT OF PORTER’S FIVE FORCES ON CYSTATIN C ASSAY MARKET
- TABLE 20 LIST OF CONFERENCES AND EVENTS (2023–2024)
- TABLE 21 CYSTATIN C ASSAY: PRICE RANGE FOR CYSTATIN C ASSAY PRODUCTS
- TABLE 22 AVERAGE SELLING PRICE OF PRODUCTS OFFERED BY KEY PLAYERS (2022)
- TABLE 23 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR END USERS
- TABLE 24 KEY BUYING CRITERIA, BY END USER
- TABLE 25 CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 26 CYSTATIN C ASSAY KITS AVAILABLE IN MARKET
- TABLE 27 CYSTATIN C ASSAY KITS MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 28 CYSTATIN C ASSAY KITS MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 29 CYSTATIN C ASSAY KITS MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 30 CYSTATIN C ASSAY KITS MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 31 CYSTATIN C ASSAY REAGENTS AVAILABLE IN MARKET
- TABLE 32 CYSTATIN C REAGENTS MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 33 CYSTATIN C ASSAY REAGENTS MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 34 CYSTATIN C ASSAY REAGENTS MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 35 CYSTATIN C ASSAY REAGENTS MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 36 CYSTATIN C ASSAY ANALYZERS AVAILABLE IN MARKET
- TABLE 37 CYSTATIN C ASSAY ANALYZERS MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 38 CYSTATIN C ASSAY ANALYZERS MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 39 CYSTATIN C ASSAY ANALYZERS MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 40 CYSTATIN C ASSAY ANALYZERS MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 41 CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 42 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 43 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 44 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 45 ENZYME-LINKED IMMUNOSORBENT ASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 46 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 47 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 48 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 49 PARTICLE-ENHANCED TURBIDIMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 50 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 51 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 52 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 53 IMMUNOFLUORESCENCE ASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 54 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 55 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 56 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 57 CHEMILUMINESCENT IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 58 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 59 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 60 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 61 PARTICLE-ENHANCED NEPHELOMETRIC IMMUNOASSAY: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 62 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 63 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 64 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 65 OTHER METHODS: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 66 CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 67 BLOOD: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 68 BLOOD: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 69 BLOOD: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 70 BLOOD: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 71 URINE: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 72 URINE: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 73 URINE: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 74 URINE: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 75 CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 76 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 77 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 78 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 79 DIAGNOSTICS: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 80 RESEARCH: CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 81 RESEARCH: CYSTATIN C ASSAY MARKET, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 82 RESEARCH: CYSTATIN C ASSAY MARKET, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 83 RESEARCH: CYSTATIN C ASSAY MARKET, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 85 CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 86 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY REGION, 2021–2028 (USD MILLION)
- TABLE 87 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 88 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 89 CYSTATIN C ASSAY MARKET FOR HOSPITALS, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 90 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY REGION, 2021–2028 (USD MILLION)
- TABLE 91 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 92 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 93 CYSTATIN C ASSAY MARKET FOR CLINICAL LABORATORIES, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 94 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY REGION, 2021–2028 (USD MILLION)
- TABLE 95 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY NORTH AMERICAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 96 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY EUROPEAN COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 97 CYSTATIN C ASSAY MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, CROS, AND ACADEMIC RESEARCH INSTITUTES, BY ASIA PACIFIC COUNTRIES, 2021–2028 (USD MILLION)
- TABLE 98 CYSTATIN C ASSAY MARKET, BY REGION, 2021–2028 (USD MILLION)
- TABLE 99 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 100 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 101 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 102 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 103 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 104 NORTH AMERICA: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 105 US: KEY MACRO INDICATORS
- TABLE 106 US: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 107 US: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 108 US: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 109 US: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 110 US: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 111 CANADA: KEY MACRO INDICATORS
- TABLE 112 CANADA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 113 CANADA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 114 CANADA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 115 CANADA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 116 CANADA: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 117 EUROPE: HEALTHCARE EXPENDITURE, BY COUNTRY (% OF GDP)
- TABLE 118 EUROPE: CYSTATIN C ASSAY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 119 EUROPE: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 120 EUROPE: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 121 EUROPE: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 122 EUROPE: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 123 EUROPE: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 124 GERMANY: KEY MACRO INDICATORS
- TABLE 125 GERMANY: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 126 GERMANY: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 127 GERMANY: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 128 GERMANY: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 129 GERMANY: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 130 FRANCE: KEY MACRO INDICATORS
- TABLE 131 FRANCE: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 132 FRANCE: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 133 FRANCE: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 134 FRANCE: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 135 FRANCE: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 136 UK: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 137 UK: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 138 UK: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 139 UK: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 140 UK: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 141 ITALY: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 142 ITALY: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 143 ITALY: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 144 ITALY: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 145 ITALY: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 146 SPAIN: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 147 SPAIN: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 148 SPAIN: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 149 SPAIN: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 150 SPAIN: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 151 REST OF EUROPE: HEALTHCARE EXPENDITURE, BY COUNTRY, 2010 VS. 2021 (% OF GDP)
- TABLE 152 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 153 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 154 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 155 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 156 REST OF EUROPE: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 157 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
- TABLE 158 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 159 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 160 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 161 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 162 ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 163 CHINA: KEY MACRO INDICATORS
- TABLE 164 CHINA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 165 CHINA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 166 CHINA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 167 CHINA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 168 CHINA: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 169 INDIA: KEY MACRO INDICATORS
- TABLE 170 INDIA: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 171 INDIA: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 172 INDIA: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 173 INDIA: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 174 INDIA: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 175 JAPAN: KEY MACRO INDICATORS
- TABLE 176 JAPAN: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 177 JAPAN: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 178 JAPAN: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 179 JAPAN: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 180 JAPAN: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 181 REST OF ASIA PACIFIC: DIABETES PREVALENCE (% OF POPULATION AGED 20–79), BY COUNTRY
- TABLE 182 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 183 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 184 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 185 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 186 REST OF ASIA PACIFIC: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 187 REST OF THE WORLD: POPULATION AGED 65 AND ABOVE (% OF TOTAL POPULATION)
- TABLE 188 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY PRODUCT, 2021–2028 (USD MILLION)
- TABLE 189 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY METHOD, 2021–2028 (USD MILLION)
- TABLE 190 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY APPLICATION, 2021–2028 (USD MILLION)
- TABLE 191 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2021–2028 (USD MILLION)
- TABLE 192 REST OF THE WORLD: CYSTATIN C ASSAY MARKET, BY END USER, 2021–2028 (USD MILLION)
- TABLE 193 OVERVIEW OF STRATEGIES ADOPTED BY KEY CYSTATIN C ASSAY MARKET PLAYERS
- TABLE 194 COMPANY FOOTPRINT ANALYSIS
- TABLE 195 COMPANY PRODUCT FOOTPRINT
- TABLE 196 COMPANY REGIONAL FOOTPRINT
- TABLE 197 CYSTATIN C ASSAY MARKET: DETAILED LIST OF KEY SMES/STARTUPS
- TABLE 198 PRODUCT LAUNCHES AND APPROVALS, 2022
- TABLE 199 DEALS, 2021
- TABLE 200 ROCHE DIAGNOSTICS LIMITED.: BUSINESS OVERVIEW
- TABLE 201 ABBOTT: BUSINESS OVERVIEW
- TABLE 202 THERMO FISHER SCIENTIFIC INC.: BUSINESS OVERVIEW
- TABLE 203 SIEMENS HEALTHCARE GMBH.: BUSINESS OVERVIEW
- TABLE 204 BIO-TECHNE.: BUSINESS OVERVIEW
- TABLE 205 AGILENT TECHNOLOGIES, INC.: BUSINESS OVERVIEW
- TABLE 206 GENTIAN DIAGNOSTICS ASA: BUSINESS OVERVIEW
- TABLE 207 ABCAM PLC.: BUSINESS OVERVIEW
- TABLE 208 GETEIN BIOTECH, INC.: BUSINESS OVERVIEW
- TABLE 209 SINO BIOLOGICAL, INC.: BUSINESS OVERVIEW
- TABLE 210 EUROLYSER DIAGNOSTICA GMBH: BUSINESS OVERVIEW
- TABLE 211 DIAZYME LABORATORIES, INC.: BUSINESS OVERVIEW
- TABLE 212 KAMIYA BIOMEDICAL COMPANY.: BUSINESS OVERVIEW
- TABLE 213 RANDOX LABORATORIES LTD..: BUSINESS OVERVIEW
- TABLE 214 DIASYS DIAGNOSTIC SYSTEMS GMBH: BUSINESS OVERVIEW
- TABLE 215 CEPHAM LIFE SCIENCES: BUSINESS OVERVIEW
- FIGURE 1 RESEARCH DESIGN
- FIGURE 2 BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY-SIDE AND DEMAND-SIDE PARTICIPANTS
- FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS
- FIGURE 4 MARKET SIZE ESTIMATION: BOTTOM-UP APPROACH
- FIGURE 5 MARKET SIZE ESTIMATION: TOP-DOWN APPROACH
- FIGURE 6 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
- FIGURE 7 DATA TRIANGULATION METHODOLOGY
- FIGURE 8 CYSTATIN C ASSAY MARKET, BY PRODUCT, 2023 VS. 2028 (USD MILLION)
- FIGURE 9 CYSTATIN C ASSAY MARKET, BY METHOD, 2023 VS. 2028 (USD MILLION)
- FIGURE 10 CYSTATIN C ASSAY MARKET, BY APPLICATION, 2023 VS. 2028 (USD MILLION)
- FIGURE 11 CYSTATIN C ASSAY MARKET, BY SAMPLE TYPE, 2023 VS. 2028 (USD MILLION)
- FIGURE 12 CYSTATIN C ASSAY MARKET, BY END USER, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 REGIONAL SNAPSHOT: CYSTATIN C ASSAY MARKET
- FIGURE 14 INCREASING PREVALENCE OF KIDNEY DISEASES AND GROWING GERIATRIC POPULATION TO DRIVE MARKET
- FIGURE 15 KITS ACCOUNTED FOR LARGEST SHARE, BY PRODUCT, IN NORTH AMERICA
- FIGURE 16 ASIA PACIFIC MARKET TO WITNESS FASTEST GROWTH DURING FORECAST PERIOD
- FIGURE 17 DEVELOPING ECONOMIES TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
- FIGURE 18 CHINA TO REGISTER HIGHEST GROWTH RATE DURING FORECAST PERIOD
- FIGURE 19 CYSTATIN C ASSAY MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 20 US: REGULATORY PROCESS FOR IVD DEVICES
- FIGURE 21 CANADA: REGULATORY PROCESS FOR IVD DEVICES IN CANADA
- FIGURE 22 VALUE CHAIN ANALYSIS: MAJOR VALUE ADDED DURING MANUFACTURING AND ASSEMBLY PHASES
- FIGURE 23 DIRECT DISTRIBUTION—PREFERRED STRATEGY FOR PROMINENT COMPANIES
- FIGURE 24 ECOSYSTEM ANALYSIS OF CYSTATIN C ASSAY MARKET
- FIGURE 25 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY END USER
- FIGURE 26 KEY BUYING CRITERIA FOR TOP END USERS
- FIGURE 27 NORTH AMERICA: CYSTATIN C ASSAY MARKET SNAPSHOT
- FIGURE 28 ASIA PACIFIC: CYSTATIN C ASSAY MARKET SNAPSHOT
- FIGURE 29 CYSTATIN C ASSAY MARKET RANKING ANALYSIS, 2022
- FIGURE 30 REVENUE ANALYSIS OF TOP FOUR PUBLIC MARKET PLAYERS
- FIGURE 31 CYSTATIN C ASSAY MARKET: COMPANY EVALUATION MATRIX (2022)
- FIGURE 32 CYSTATIN C ASSAY MARKET: COMPANY EVALUATION MATRIX FOR STARTUPS/SMES (2022)
- FIGURE 33 ROCHE DIAGNOSTICS LIMITED.: COMPANY SNAPSHOT
- FIGURE 34 ABBOTT: COMPANY SNAPSHOT
- FIGURE 35 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT
- FIGURE 36 SIEMENS HEALTHCARE GMBH.: COMPANY SNAPSHOT
- FIGURE 37 BIO-TECHNE.: COMPANY SNAPSHOT
- FIGURE 38 AGILENT TECHNOLOGIES, INC. COMPANY SNAPSHOT
- FIGURE 39 GENTIAN DIAGNOSTICS ASA: COMPANY SNAPSHOT
- FIGURE 40 ABCAM PLC.: COMPANY SNAPSHOT
- FIGURE 41 GETEIN BIOTECH, INC.: COMPANY SNAPSHOT
- FIGURE 42 SINO BIOLOGICAL, INC.: COMPANY SNAPSHOT
The study involved four major activities in estimating the current size of the cystatin C assay market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate the market size of the segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for this study.
Primary Research
Extensive primary research was conducted after acquiring knowledge about the global market scenario through secondary research. Primary interviews were conducted from both the demand (Pathologist, Head of laboratory, Microbiologist) and supply sides (cystatin C assay manufacturers and distributors).
The following is a breakdown of the primary respondents:
Breakdown of Primary Participants:
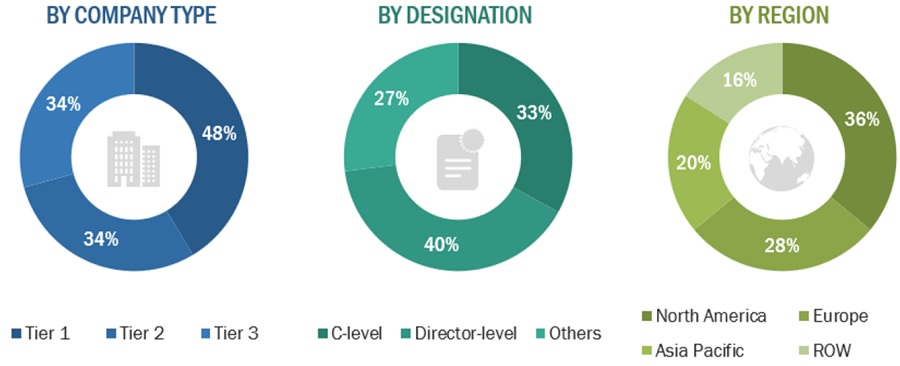
Note 1: Others include sales managers, marketing managers, and product managers.
Note 2. Tiers of companies are defined based on their total revenue. As of 2022: Tier 1 = >USD 5 billion, Tier 2 = USD 500 million to USD 5 billion, and Tier 3 = <USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the cystatin C assay market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and markets have been identified through extensive secondary research.
- The industry’s supply chain and market size, in terms of value, have been determined through primary and secondary research processes.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
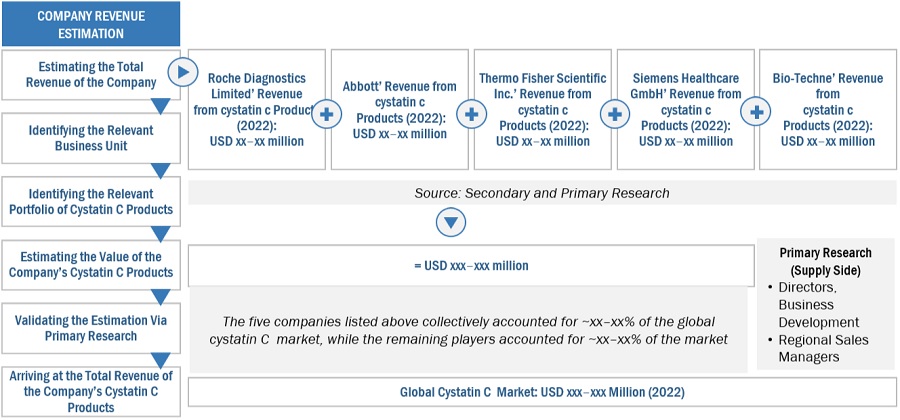
To know about the assumptions considered for the study, Request for Free Sample Report

Data Triangulation
After arriving at the overall market size—using the market size estimation processes—the market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, the data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the cystatin C assay industry.
Report Objectives
- To define, describe, and forecast the global cystatin C assay market based on product, method, applications, sample type, end users, and region.
- To provide detailed information regarding the major factors influencing the growth of the market (drivers, opportunities, challenges, Restraints)
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders.
- To forecast the size of market segments with respect to four main regions—North America, Europe, Asia Pacific, Rest of the World.
- To strategically profile key players and comprehensively analyze their product portfolios, market shares, and core competencies3.
- To track and analyze competitive developments such as acquisitions, agreements, new product launches, and partnerships in the cystatin C assay market. market m
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
- Product Analysis: Product matrix, which gives a detailed comparison of the product portfolios of each company.
- Geographic Analysis: Further breakdown of the European cystatin C assay market into specific countries.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes—the market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, the data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the cystatin C assay industry.
Report Objectives
- To define, describe, and forecast the global cystatin C assay market based on product, method, applications, sample type, end users, and region.
- To provide detailed information regarding the major factors influencing the growth of the market (drivers, opportunities, challenges, Restraints)
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders.
- To forecast the size of market segments with respect to four main regions—North America, Europe, Asia Pacific, Rest of the World.
- To strategically profile key players and comprehensively analyze their product portfolios, market shares, and core competencies3.
- To track and analyze competitive developments such as acquisitions, agreements, new product launches, and partnerships in the cystatin C assay market. market m
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
- Product Analysis: Product matrix, which gives a detailed comparison of the product portfolios of each company.
- Geographic Analysis: Further breakdown of the European cystatin C assay market into specific countries.















Growth opportunities and latent adjacency in Cystatin C Assay Market