2
RESEARCH METHODOLOGY
62
5
MARKET OVERVIEW
Emerging markets and tech innovations drive growth despite regulatory and cost challenges.
86
5.2.1.1
INCREASING GERIATRIC POPULATION AND SUBSEQUENT RISE IN CHRONIC DISEASES
5.2.1.2
GROWING AWARENESS OF EARLY DISEASE DIAGNOSIS IN EMERGING ECONOMIES
5.2.1.3
EMERGENCE OF RAPID POC TECHNOLOGIES AND RISING ADOPTION OF AUTOMATED ANALYZERS
5.2.1.4
GROWING PREFERENCE FOR PERSONALIZED MEDICINES
5.2.2.1
UNFAVORABLE REIMBURSEMENT SCENARIO
5.2.2.2
STRINGENT REGULATORY REQUIREMENTS
5.2.2.3
HIGH COST OF DIAGNOSTIC EQUIPMENT
5.2.3.1
INTRODUCTION OF DISEASE-SPECIFIC BIOMARKERS AND TESTS
5.2.3.2
INCREASING SIGNIFICANCE OF COMPANION DIAGNOSTICS
5.2.3.3
GROWTH OPPORTUNITIES IN DEVELOPING COUNTRIES
5.2.3.4
IMPROVEMENTS IN IMMUNOASSAY DIAGNOSTIC TECHNOLOGIES
5.2.3.5
DIGITALIZATION TREND
5.2.4.1
OPERATIONAL BARRIERS
5.2.4.2
DATA PRIVACY AND CYBERSECURITY RISKS
5.3
TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
5.4.1
AVERAGE SELLING PRICE TREND, BY KEY PLAYER
5.4.2
AVERAGE SELLING PRICE TREND, BY REGION
5.4.3
AVERAGE SELLING PRICE TREND, BY PRODUCT
5.5.1
RESEARCH & DEVELOPMENT
5.5.2
RAW MATERIAL SUPPLIERS
5.5.4
DISTRIBUTION, MARKETING, AND SALES
5.5.6
POST-SALES SERVICES
5.6
SUPPLY CHAIN ANALYSIS
5.6.1
PROMINENT COMPANIES
5.6.2
SMALL & MEDIUM-SIZED ENTERPRISES
5.8
INVESTMENT AND FUNDING SCENARIO
5.9.2
COMPLEMENTARY TECHNOLOGIES
5.9.2.1
IMMUNOHISTOCHEMISTRY
5.11.1
IMPORT DATA FOR HS CODE 382200
5.11.2
EXPORT DATA FOR HS CODE 382200
5.12
KEY CONFERENCES AND EVENTS, 2025–2026
5.13.1
CASE STUDY 1: LOCALIZED STRATEGY TO TAP MARKET POTENTIAL IN EUROPE
5.13.2
CASE STUDY 2: ENHANCED COMPANY FOOTPRINT IN COMPETITIVE INDIAN MARKET
5.13.3
CASE STUDY 3: CUSTOMER-CENTRIC DIFFERENTIATION FOR PRODUCT OPTIMIZATION AND PREMIUM PRICING
5.14
REGULATORY LANDSCAPE
5.14.1
REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.15
REGULATORY FRAMEWORK
5.15.4
MIDDLE EAST & AFRICA
5.16
PORTER’S FIVE FORCES ANALYSIS
5.16.1
BARGAINING POWER OF SUPPLIERS
5.16.2
BARGAINING POWER OF BUYERS
5.16.3
THREAT OF NEW ENTRANTS
5.16.4
THREAT OF SUBSTITUTES
5.16.5
INTENSITY OF COMPETITIVE RIVALRY
5.17
KEY STAKEHOLDERS AND BUYING CRITERIA
5.17.1
KEY STAKEHOLDERS IN BUYING PROCESS
5.18
RECENT POLICY CHANGES IN US MARKET AND THEIR POTENTIAL IMPACT ON APPROVAL PROCESS
5.19
INFLUENCE OF TARIFFS ON SUPPLY CHAIN RESILIENCE OF MEDTECH COMPANIES
5.20
GROWTH OF DECENTRALIZED TESTING VS. ADOPTION OF COST-EFFECTIVE CENTRALIZED TESTING
5.21
IMPACT OF AI/GENERATIVE AI ON IN VITRO DIAGNOSTICS MARKET
5.21.2
MARKET POTENTIAL OF AI IN IVD DEVICES
5.21.4
KEY COMPANIES IMPLEMENTING AI
5.21.5
FUTURE OF GENERATIVE AI IN IN VITRO DIAGNOSTICS MARKET
6
IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 21 Data Tables
141
6.2.1
RISE IN TEST VOLUMES ACROSS CLINICAL LABS, HOSPITALS, AND POINT-OF-CARE SETTINGS TO AID GROWTH
6.3.1
GROWING TREND OF AUTOMATION TO DRIVE MARKET
6.4
DATA MANAGEMENT SOFTWARE & SERVICES
6.4.1
INCREASING SHIFT TOWARD CLOUD-BASED PLATFORMS AND REMOTE DATA ACCESS TO STIMULATE GROWTH
7
IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 198 Data Tables
156
7.2.1
ENZYME-LINKED IMMUNOSORBENT ASSAYS (ELISA)
7.2.1.1
INCREASING USE OF IMMUNOASSAYS IN CANCER, INFECTIOUS DISEASE TESTING, AND THERAPEUTIC DRUG MONITORING TO AID GROWTH
7.2.2
CHEMILUMINESCENCE IMMUNOASSAYS
7.2.2.1
NEED FOR MINIMAL HUMAN INTERVENTION TO SUPPORT GROWTH
7.2.3
IMMUNOFLUORESCENCE ASSAYS
7.2.3.1
GROWING INTRODUCTION OF INNOVATIVE DIAGNOSTIC PLATFORMS TO DRIVE MARKET
7.2.4.1
NEED FOR RAPID DIAGNOSTIC TESTS TO EXPEDITE GROWTH
7.2.5
ENZYME-LINKED IMMUNOSPOT ASSAYS (ELISPOT)
7.2.5.1
NEED FOR EARLY DETECTION AND MONITORING OF IMMUNE-RELATED DISORDERS TO AMPLIFY GROWTH
7.2.6.1
INCREASING FOCUS ON BIOMARKER DISCOVERY AND PERSONALIZED TREATMENT TO ADVANCE GROWTH
7.2.7
OTHER IMMUNOASSAY TECHNOLOGIES
7.3.1
BASIC METABOLIC PANELS
7.3.1.1
INCREASING INCIDENCE OF DIABETES, KIDNEY DISORDERS, AND HYPERTENSION TO PROMOTE GROWTH
7.3.2.1
RISING PREVALENCE OF CIRRHOSIS AND VIRAL HEPATITIS TO FOSTER GROWTH
7.3.3.1
GROWING AWARENESS FOR PREVENTIVE HEALTH CHECK-UPS TO BOOST MARKET
7.3.4.1
RISING PREVALENCE OF OBESITY TO ACCELERATE GROWTH
7.3.5
THYROID FUNCTION PROFILES
7.3.5.1
HIGH INCIDENCE OF THYROID-RELATED DISORDERS TO SUPPORT GROWTH
7.3.6.1
GROWING SHIFT TOWARD HOME CARE AND REMOTE PATIENT MONITORING TO FUEL MARKET
7.3.7
SPECIALTY CHEMICAL TESTS
7.3.7.1
RISING DEMAND FOR SPECIALIZED TESTS TO CONTRIBUTE TO GROWTH
7.4
MOLECULAR DIAGNOSTICS
7.4.1
POLYMERASE CHAIN REACTION
7.4.1.1
HIGH ACCURACY AND SPEED IN DETECTING PATHOGENS TO AID GROWTH
7.4.2
ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY
7.4.2.1
GROWING FOCUS ON RAPID, ONSITE MOLECULAR DIAGNOSTICS TO PROPEL MARKET
7.4.3
DNA SEQUENCING & NEXT-GENERATION SEQUENCING
7.4.3.1
GROWING APPLICATIONS IN RESEARCH, DRUG DISCOVERY, AND MOLECULAR DIAGNOSTICS TO BOOST MARKET
7.4.4
IN SITU HYBRIDIZATION
7.4.4.1
RISING PREVALENCE OF CANCER AND GENETIC DISORDERS TO ASSIST GROWTH
7.4.5.1
HIGH RESOLUTION AND THROUGHPUT CAPABILITIES TO FAVOR GROWTH
7.4.6
OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES
7.5.1
ONGOING TECHNOLOGICAL ADVANCEMENTS IN BLOOD GLUCOSE SELF-MONITORING DEVICES TO AID GROWTH
7.6.1
GROWING FOCUS ON STEM CELL RESEARCH TO BOOST MARKET
7.7.1
RISING PREVALENCE OF MICROBIAL INFECTIONS TO SUSTAIN GROWTH
7.8
COAGULATION & HEMOSTASIS
7.8.1
GROWING CASES OF PULMONARY EMBOLISM, HEMOPHILIA, LIVER DISEASES TO DRIVE MARKET
7.9.1
GROWING SURGICAL INTERVENTIONS AND RISE IN ELDERLY POPULATION TO FUEL MARKET
7.10.1
RISING INCIDENCE OF URINARY TRACT INFECTIONS TO SUPPORT GROWTH
7.11
CHROMATOGRAPHY & MASS SPECTROMETRY
7.11.1
INCREASING APPLICATIONS IN DISEASE SCREENING AND DIAGNOSTICS TO AID GROWTH
8
IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 27 Data Tables
270
8.2
BLOOD, SERUM, AND PLASMA SPECIMENS
8.2.1
GROWING SHIFT TOWARD DECENTRALIZED TESTING TO PROPEL MARKET
8.3.1
GROWING ADVANCEMENTS IN MOLECULAR DIAGNOSTICS TO BOOST MARKET
8.4.1
RISING INCIDENCE OF URINARY TRACT INFECTIONS TO ACCELERATE GROWTH
9
IN VITRO DIAGNOSTICS MARKET, BY SITE OF TESTING
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 15 Data Tables
286
9.2.1
INCREASING FOCUS ON EARLY AND ACCURATE DISEASE DETECTION TO ENCOURAGE GROWTH
9.3.1
RISING DEMAND FOR DECENTRALIZED HEALTHCARE TO CONTRIBUTE TO GROWTH
10
IN VITRO DIAGNOSTICS MARKET, BY APPLICATION
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 84 Data Tables
299
10.2.1
INCREASING PREVALENCE OF VIRAL, BACTERIAL, AND PARASITIC INFECTIONS TO PROMOTE GROWTH
10.3.1
RISING GLOBAL BURDEN OF CANCER TO EXPEDITE GROWTH
10.4.1
INCREASING PREVALENCE OF DIABETES AND THYROID-RELATED DISORDERS TO BOLSTER GROWTH
10.5.1
GROWING USE OF IMMUNOASSAYS IN CARDIOVASCULAR DISEASE DETECTION TO DRIVE MARKET
10.6.1
RISING INCIDENCE OF HIV AND SYPHILIS TO SUPPORT GROWTH
10.7.1
INCREASING CLINICAL UTILITY OF GENETIC TESTS ACROSS ONCOLOGY, RARE DISEASES, AND REPRODUCTIVE HEALTH TO FOSTER GROWTH
10.8.1
RISE IN AUTOIMMUNE CONDITIONS GLOBALLY TO FACILITATE GROWTH
10.9.1
INCREASING AWARENESS ABOUT ALLERGIES TO AMPLIFY GROWTH
10.10
DRUG MONITORING & TESTING
10.10.1
INCREASING ILLICIT DRUG CONSUMPTION TO SUSTAIN GROWTH
10.11
BONE & MINERAL DISORDERS
10.11.1
RISING CASES OF OSTEOPOROSIS TO FUEL MARKET
10.12
COAGULATION TESTING
10.12.1
INCREASING PREVALENCE OF HEMOPHILIA TO ADVANCE GROWTH
10.13.1
RISING NUMBER OF SURGICAL PROCEDURES TO SPEED UP GROWTH
11
IN VITRO DIAGNOSTICS MARKET, BY END USER
Market Size & Growth Rate Forecast Analysis to 2030 in USD Million | 56 Data Tables
349
11.2.1
EXPANDING HEALTHCARE INFRASTRUCTURE TO SPEED UP GROWTH
11.3
CLINICAL LABORATORIES
11.3.1
LARGE REFERENCE LABORATORIES
11.3.1.1
GROWING TEST VOLUME AND COMPLEXITY OF DIAGNOSTIC TESTING TO PROPEL MARKET
11.3.2
SMALL AND MEDIUM-SIZED LABORATORIES
11.3.2.1
INCREASING SHIFT TOWARD COMMUNITY-BASED AND POINT-OF-CARE SETTINGS TO FOSTER GROWTH
11.4.1
NEED TO ADDRESS PUBLIC HEALTH AND SAFETY CHALLENGES TO AID GROWTH
11.5.1
GROWING INCLINATION TOWARD SELF-DIAGNOSIS AND MONITORING TO BOOST MARKET
11.6
PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
11.6.1
INCREASING USE OF IVD PRODUCTS IN CLINICAL DRUG DEVELOPMENT AND COMPANION DIAGNOSTICS TO DRIVE MARKET
11.7.1
RISING INDUSTRY-ACADEMIA COLLABORATIONS FOR PRODUCT DEVELOPMENT TO AUGMENT GROWTH
12
IN VITRO DIAGNOSTICS MARKET, BY REGION
Comprehensive coverage of 8 Regions with country-level deep-dive of 18 Countries | 317 Data Tables.
382
12.2.1
MACROECONOMIC OUTLOOK FOR NORTH AMERICA
12.2.2.1
ESTABLISHED REIMBURSEMENT FRAMEWORK AND FAVORABLE POLICIES FOR IVD PROVIDERS TO DRIVE MARKET
12.2.3.1
FAVORABLE FUNDING INITIATIVES FOR EARLY DISEASE DIAGNOSIS TO FUEL MARKET
12.3.1
MACROECONOMIC OUTLOOK FOR EUROPE
12.3.2.1
INCREASING INVESTMENTS IN CLINICAL DIAGNOSTICS RESEARCH TO AID GROWTH
12.3.3.1
HIGH HEALTHCARE EXPENDITURE AND RISING INVESTMENTS IN GENOMIC MEDICINE TO DRIVE MARKET
12.3.4.1
RISING ADOPTION OF GENOME-BASED TESTING TO ENCOURAGE GROWTH
12.3.5.1
GROWING GERIATRIC POPULATION AND SUBSEQUENT RISE IN CHRONIC CONDITIONS TO BOOST MARKET
12.3.6.1
INCREASING ADOPTION OF TECHNOLOGICALLY ADVANCED IMMUNOASSAY SYSTEMS TO EXPEDITE GROWTH
12.3.7.1
HIGH INCIDENCE OF RESPIRATORY INFECTIOUS DISEASES TO BOOST MARKET
12.3.8.1
GROWING PREVALENCE OF CHRONIC DISEASES TO PROPEL MARKET
12.4.1
MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
12.4.2.1
INCREASING RESEARCH INVESTMENTS FOR IMMUNOASSAYS TO ENCOURAGE GROWTH
12.4.3.1
GROWING FOCUS ON PREVENTIVE CARE TO BOOST MARKET
12.4.4.1
RISING INCIDENCE OF DIABETES AND CANCER TO AUGMENT GROWTH
12.4.5.1
RISING HEALTHCARE SPENDING FOR INNOVATIVE IVD TECHNOLOGIES TO AMPLIFY GROWTH
12.4.6.1
INCREASING INCIDENCE OF CHRONIC DISEASES AND RISING BLOOD DONATIONS TO FUEL MARKET
12.4.7.1
RISING TECHNOLOGICAL ADVANCEMENTS AND AUTOMATION TO STIMULATE GROWTH
12.4.8
REST OF ASIA PACIFIC
12.5.1
MACROECONOMIC OUTLOOK FOR LATIN AMERICA
12.5.2.1
RISING PREVALENCE OF DIABETES TO SUSTAIN GROWTH
12.5.3.1
GROWING ESTABLISHMENT OF CLINICAL LABORATORIES TO FUEL MARKET
12.5.4
REST OF LATIN AMERICA
12.6
MIDDLE EAST & AFRICA
12.6.1
GROWING FOCUS ON PRENATAL AND CANCER TESTING TO DRIVE MARKET
12.6.2
MIDDLE EAST & AFRICA: MACROECONOMIC OUTLOOK
12.7.1
GCC COUNTRIES: MACROECONOMIC OUTLOOK
12.7.2.1
RISING GOVERNMENT HEALTHCARE EXPENDITURE TO BOOST MARKET
12.7.3.1
IMPROVEMENTS IN HEALTHCARE INFRASTRUCTURE TO SUPPORT GROWTH
12.7.4
OTHER GCC COUNTRIES
13
COMPETITIVE LANDSCAPE
Uncover strategic maneuvers and market positioning of industry leaders and rising startups.
561
13.2
KEY PLAYER STRATEGIES/RIGHT TO WIN
13.3
OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS
13.4
REVENUE ANALYSIS, 2022–2024
13.5
MARKET SHARE ANALYSIS, 2024
13.6
COMPANY VALUATION AND FINANCIAL METRICS
13.7
BRAND/PRODUCT COMPARISON
13.8
COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
13.8.5
COMPANY FOOTPRINT: KEY PLAYERS, 2024
13.8.5.1
COMPANY FOOTPRINT
13.8.5.2
REGION FOOTPRINT
13.8.5.3
PRODUCT & SERVICE FOOTPRINT
13.8.5.4
TECHNOLOGY FOOTPRINT
13.8.5.5
END-USER FOOTPRINT
13.9
COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
13.9.1
PROGRESSIVE COMPANIES
13.9.2
RESPONSIVE COMPANIES
13.9.5
COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
13.9.5.1
DETAILED LIST OF KEY STARTUPS/SMES
13.9.5.2
COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
13.10
COMPETITIVE SCENARIO
13.10.1
PRODUCT LAUNCHES AND APPROVALS
13.11
POTENTIAL FUTURISTIC STRATEGIES OF LEADING PLAYERS OPERATING IN IVD MARKET
13.11.1
PRODUCT LAUNCHES AND APPROVALS
14
COMPANY PROFILES
In-depth Company Profiles of Leading Market Players with detailed Business Overview, Product and Service Portfolio, Recent Developments, and Unique Analyst Perspective (MnM View)
585
14.1.1.1
BUSINESS OVERVIEW
14.1.1.2
PRODUCTS/SERVICES OFFERED
14.1.1.3
RECENT DEVELOPMENTS
14.1.1.3.1
PRODUCT LAUNCHES AND APPROVALS
14.1.1.4.2
STRATEGIC CHOICES
14.1.1.4.3
WEAKNESSES AND COMPETITIVE THREATS
14.1.2
F. HOFFMANN-LA ROCHE LTD
14.1.4
SIEMENS HEALTHINEERS AG
14.1.5
THERMO FISHER SCIENTIFIC INC.
14.1.8
BIO-RAD LABORATORIES, INC.
14.1.10
SYSMEX CORPORATION
14.1.12
BECTON, DICKINSON AND COMPANY
14.1.13
AGILENT TECHNOLOGIES, INC.
14.1.18
QUIDELORTHO CORPORATION
14.2.4
MENARINI SILICON BIOSYSTEMS
14.2.6
GENSPEED BIOTECH GMBH
14.2.8
CARIS LIFE SCIENCES
14.2.10
ACCELERATE DIAGNOSTICS, INC.
14.2.12
J. MITRA & CO. PVT. LTD.
14.2.13
EPITOPE DIAGNOSTICS, INC.
14.2.14
BOSTER BIOLOGICAL TECHNOLOGY
14.2.15
ENZO BIOCHEM INC.
14.2.16
GENETIC SIGNATURES
14.2.17
SAVYON DIAGNOSTICS
14.2.18
TRIVITRON HEALTHCARE
14.2.20
CREATIVE DIAGNOSTICS
14.2.21
INBIOS INTERNATIONAL, INC.
14.2.22
MACCURA BIOTECHNOLOGY CO., LTD.
14.2.23
LUYE LIFE SCIENCES GROUP
15.2
KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
15.3
CUSTOMIZATION OPTIONS
TABLE 1
IN VITRO DIAGNOSTICS MARKET: INCLUSIONS AND EXCLUSIONS
TABLE 2
IN VITRO DIAGNOSTICS MARKET: RISK ASSESSMENT
TABLE 3
ESTIMATED INCREASE IN GERIATRIC POPULATION, BY REGION, 2022−2050
TABLE 4
IN VITRO DIAGNOSTICS MARKET: PRODUCT LAUNCHES, 2022−2024
TABLE 5
APPLICATION OF CANCER BIOMARKERS IN CLINICAL PRACTICE
TABLE 6
RECENT DEVELOPMENTS IN IVD MARKET, 2021–2023
TABLE 7
AVERAGE SELLING PRICE TREND OF IVD PRODUCTS, BY KEY PLAYER, 2022–2024 (USD)
TABLE 8
AVERAGE SELLING PRICE TREND OF CLINICAL CHEMISTRY ANALYZERS, BY REGION, 2022–2024 (USD)
TABLE 9
AVERAGE SELLING PRICE TREND OF IVD PRODUCTS, BY TYPE, 2022–2024 (USD)
TABLE 10
IN VITRO DIAGNOSTICS MARKET: ROLE OF COMPANIES IN ECOSYSTEM
TABLE 11
IN VITRO DIAGNOSTICS MARKET: INNOVATIONS AND PATENT REGISTRATIONS, 2022−2024
TABLE 12
IMPORT DATA FOR HS CODE 382200, BY COUNTRY, 2019–2024 (USD MILLION)
TABLE 13
EXPORT DATA FOR HS CODE 382200, BY COUNTRY, 2019–2024 (USD MILLION)
TABLE 14
IN VITRO DIAGNOSTICS MARKET: KEY CONFERENCES AND EVENTS, 2025−2026
TABLE 15
NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 16
EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 17
ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 18
LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 19
REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 20
US: CLASSIFICATION OF IVD DEVICES
TABLE 21
EUROPE: CLASSIFICATION OF IVD DEVICES
TABLE 22
JAPAN: CLASSIFICATION OF IVD REAGENTS IN JAPAN
TABLE 23
JAPAN: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 24
CHINA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 25
SOUTH KOREA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 26
INDONESIA: REGISTRATION PROCESS FOR IVD DEVICES
TABLE 27
RUSSIA: CLASSIFICATION OF IVD DEVICES
TABLE 28
SAUDI ARABIA: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 29
MEXICO: TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS
TABLE 30
IN VITRO DIAGNOSTICS MARKET: PORTER’S FIVE FORCES ANALYSIS
TABLE 31
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF IVD PRODUCTS (%)
TABLE 32
KEY BUYING CRITERIA, BY PRODUCT & SERVICE
TABLE 33
US: OVERVIEW OF CERTAIN KEY CATEGORIES OF IVD DEVICES
TABLE 34
TARIFF RATES IMPOSED BY US, AS OF APRIL 2025
TABLE 35
COMPARATIVE ANALYSIS OF CENTRALIZED VS. DECENTRALIZED TESTING
TABLE 36
IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 37
IN VITRO DIAGNOSTICS MARKET FOR REAGENTS & KITS, BY REGION, 2022–2030 (USD MILLION)
TABLE 38
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 39
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 40
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 41
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 42
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 43
KEY IVD INSTRUMENTS AVAILABLE WORLDWIDE
TABLE 44
IN VITRO DIAGNOSTICS MARKET FOR INSTRUMENTS, BY REGION, 2022–2030 (USD MILLION)
TABLE 45
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR INSTRUMENTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 46
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR INSTRUMENTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 47
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR INSTRUMENTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 48
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR INSTRUMENTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 49
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR INSTRUMENTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 50
KEY DATA MANAGEMENT SOFTWARE SOLUTIONS AVAILABLE WORLDWIDE
TABLE 51
IN VITRO DIAGNOSTICS MARKET FOR DATA MANAGEMENT SOFTWARE & SERVICES, BY REGION, 2022–2030 (USD MILLION)
TABLE 52
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DATA MANAGEMENT SOFTWARE & SERVICES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 53
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR DATA MANAGEMENT SOFTWARE & SERVICES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 54
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR DATA MANAGEMENT SOFTWARE & SERVICES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 55
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DATA MANAGEMENT SOFTWARE & SERVICES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 56
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR DATA MANAGEMENT SOFTWARE & SERVICES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 57
IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 58
KEY IMMUNOASSAY ANALYZERS AVAILABLE WORLDWIDE
TABLE 59
IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 60
IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY REGION, 2022–2030 (USD MILLION)
TABLE 61
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 62
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 63
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 64
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 65
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 66
KEY ELISA ANALYZERS AVAILABLE WORLDWIDE
TABLE 67
IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSORBENT ASSAYS, BY REGION, 2022–2030 (USD MILLION)
TABLE 68
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSORBENT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 69
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSORBENT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 70
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSORBENT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 71
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSORBENT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 72
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSORBENT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 73
KEY CHEMILUMINESCENCE IMMUNOASSAY SYSTEMS AVAILABLE WORLDWIDE
TABLE 74
IN VITRO DIAGNOSTICS MARKET FOR CHEMILUMINESCENCE IMMUNOASSAYS, BY REGION, 2022–2030 (USD MILLION)
TABLE 75
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CHEMILUMINESCENCE IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 76
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CHEMILUMINESCENCE IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 77
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CHEMILUMINESCENCE IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 78
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CHEMILUMINESCENCE IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 79
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CHEMILUMINESCENCE IMMUNOASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 80
IN VITRO DIAGNOSTICS MARKET FOR IMMUNOFLUORESCENCE ASSAYS, BY REGION, 2022–2030 (USD MILLION)
TABLE 81
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOFLUORESCENCE ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 82
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOFLUORESCENCE ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 83
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOFLUORESCENCE ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 84
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOFLUORESCENCE ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 85
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOFLUORESCENCE ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 86
KEY RAPID TESTS AVAILABLE WORLDWIDE
TABLE 87
IN VITRO DIAGNOSTICS MARKET FOR RAPID TESTS, BY REGION, 2022–2030 (USD MILLION)
TABLE 88
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR RAPID TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 89
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR RAPID TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 90
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR RAPID TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 91
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR RAPID TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 92
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR RAPID TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 93
IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSPOT ASSAYS, BY REGION, 2022–2030 (USD MILLION)
TABLE 94
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSPOT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 95
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSPOT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 96
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSPOT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 97
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSPOT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 98
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ENZYME-LINKED IMMUNOSPOT ASSAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 99
KEY WESTERN BLOTTING SYSTEMS AVAILABLE WORLDWIDE
TABLE 100
IN VITRO DIAGNOSTICS MARKET FOR WESTERN BLOTTING, BY REGION, 2022–2030 (USD MILLION)
TABLE 101
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR WESTERN BLOTTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 102
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR WESTERN BLOTTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 103
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR WESTERN BLOTTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 104
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR WESTERN BLOTTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 105
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR WESTERN BLOTTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 106
IN VITRO DIAGNOSTICS MARKET FOR OTHER IMMUNOASSAY TECHNOLOGIES, BY REGION, 2022–2030 (USD MILLION)
TABLE 107
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER IMMUNOASSAY TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 108
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR OTHER IMMUNOASSAY TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 109
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR OTHER IMMUNOASSAY TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 110
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER IMMUNOASSAY TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 111
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR OTHER IMMUNOASSAY TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 112
CLINICAL CHEMISTRY ANALYZERS FOR HIGH AND MID-VOLUME LABORATORIES AVAILABLE WORLDWIDE
TABLE 113
CLINICAL CHEMISTRY ANALYZERS FOR LOW-VOLUME LABORATORIES AVAILABLE WORLDWIDE
TABLE 114
IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 115
IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY REGION, 2022–2030 (USD MILLION)
TABLE 116
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 117
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 118
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 119
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 120
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 121
IN VITRO DIAGNOSTICS MARKET FOR BASIC METABOLIC PANELS, BY REGION, 2022–2030 (USD MILLION)
TABLE 122
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BASIC METABOLIC PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 123
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BASIC METABOLIC PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 124
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BASIC METABOLIC PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 125
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BASIC METABOLIC PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 126
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BASIC METABOLIC PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 127
IN VITRO DIAGNOSTICS MARKET FOR LIVER PANELS, BY REGION, 2022–2030 (USD MILLION)
TABLE 128
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LIVER PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 129
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR LIVER PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 130
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR LIVER PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 131
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LIVER PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 132
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR LIVER PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 133
IN VITRO DIAGNOSTICS MARKET FOR RENAL PROFILES, BY REGION, 2022–2030 (USD MILLION)
TABLE 134
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR RENAL PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 135
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR RENAL PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 136
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR RENAL PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 137
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR RENAL PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 138
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR RENAL PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 139
IN VITRO DIAGNOSTICS MARKET FOR LIPID PROFILES, BY REGION, 2022–2030 (USD MILLION)
TABLE 140
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LIPID PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 141
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR LIPID PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 142
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR LIPID PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 143
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LIPID PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 144
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR LIPID PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 145
IN VITRO DIAGNOSTICS MARKET FOR THYROID FUNCTION PROFILES, BY REGION, 2022–2030 (USD MILLION)
TABLE 146
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR THYROID FUNCTION PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 147
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR THYROID FUNCTION PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 148
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR THYROID FUNCTION PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 149
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR THYROID FUNCTION PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 150
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR THYROID FUNCTION PROFILES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 151
REFERENCE RANGES AND ABNORMAL CONDITIONS FOR KEY ELECTROLYTES
TABLE 152
IN VITRO DIAGNOSTICS MARKET FOR ELECTROLYTE PANELS, BY REGION, 2022–2030 (USD MILLION)
TABLE 153
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ELECTROLYTE PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 154
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ELECTROLYTE PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 155
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ELECTROLYTE PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 156
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ELECTROLYTE PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 157
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ELECTROLYTE PANELS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 158
IN VITRO DIAGNOSTICS MARKET FOR SPECIALTY CHEMICAL TESTS, BY REGION, 2022–2030 (USD MILLION)
TABLE 159
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR SPECIALTY CHEMICAL TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 160
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR SPECIALTY CHEMICAL TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 161
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR SPECIALTY CHEMICAL TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 162
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR SPECIALTY CHEMICAL TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 163
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR SPECIALTY CHEMICAL TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 164
KEY AUTOMATED MOLECULAR DIAGNOSTIC PLATFORMS AVAILABLE WORLDWIDE
TABLE 165
IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 166
IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY REGION, 2022–2030 (USD MILLION)
TABLE 167
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 168
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 169
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 170
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 171
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 172
KEY POLYMERASE CHAIN REACTION INSTRUMENTS AVAILABLE WORLDWIDE
TABLE 173
IN VITRO DIAGNOSTICS MARKET FOR POLYMERASE CHAIN REACTION, BY REGION, 2022–2030 (USD MILLION)
TABLE 174
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 175
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 176
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 177
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 178
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 179
KEY ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY INSTRUMENTS AVAILABLE WORLDWIDE
TABLE 180
IN VITRO DIAGNOSTICS MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY REGION, 2022–2030 (USD MILLION)
TABLE 181
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 182
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 183
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 184
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 185
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 186
KEY NEXT-GENERATION SEQUENCING-BASED INSTRUMENTS AVAILABLE WORLDWIDE
TABLE 187
IN VITRO DIAGNOSTICS MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY REGION, 2022–2030 (USD MILLION)
TABLE 188
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 189
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 190
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 191
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 192
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 193
IN VITRO DIAGNOSTICS MARKET FOR IN SITU HYBRIDIZATION, BY REGION, 2022–2030 (USD MILLION)
TABLE 194
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 195
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 196
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 197
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 198
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 199
IN VITRO DIAGNOSTICS MARKET FOR DNA MICROARRAYS, BY REGION, 2022–2030 (USD MILLION)
TABLE 200
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 201
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 202
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 203
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 204
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 205
IN VITRO DIAGNOSTICS MARKET FOR OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES, BY REGION, 2022–2030 (USD MILLION)
TABLE 206
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 207
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 208
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 209
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 210
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR OTHER MOLECULAR DIAGNOSTIC TECHNOLOGIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 211
DIABETES-RELATED HEALTH EXPENDITURE PER PERSON, 2021 VS. 2030 VS. 2045 (USD)
TABLE 212
IN VITRO DIAGNOSTICS MARKET FOR GLUCOSE MONITORING, BY REGION, 2022–2030 (USD MILLION)
TABLE 213
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR GLUCOSE MONITORING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 214
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR GLUCOSE MONITORING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 215
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR GLUCOSE MONITORING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 216
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR GLUCOSE MONITORING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 217
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR GLUCOSE MONITORING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 218
IN VITRO DIAGNOSTICS MARKET FOR HEMATOLOGY, BY REGION, 2022–2030 (USD MILLION)
TABLE 219
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 220
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 221
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 222
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 223
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 224
IN VITRO DIAGNOSTICS MARKET FOR MICROBIOLOGY, BY REGION, 2022–2030 (USD MILLION)
TABLE 225
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MICROBIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 226
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR MICROBIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 227
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR MICROBIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 228
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MICROBIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 229
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR MICROBIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 230
IN VITRO DIAGNOSTICS MARKET FOR COAGULATION & HEMOSTASIS, BY REGION, 2022–2030 (USD MILLION)
TABLE 231
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION & HEMOSTASIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 232
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION & HEMOSTASIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 233
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION & HEMOSTASIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 234
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION & HEMOSTASIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 235
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION & HEMOSTASIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 236
KEY BLOOD GAS ANALYZERS AVAILABLE WORLDWIDE
TABLE 237
IN VITRO DIAGNOSTICS MARKET FOR BLOOD GAS ANALYZERS, BY REGION, 2022–2030 (USD MILLION)
TABLE 238
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GAS ANALYZERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 239
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GAS ANALYZERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 240
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GAS ANALYZERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 241
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GAS ANALYZERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 242
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GAS ANALYZER, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 243
IN VITRO DIAGNOSTICS MARKET FOR URINALYSIS, BY REGION, 2022–2030 (USD MILLION)
TABLE 244
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR URINALYSIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 245
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR URINALYSIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 246
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR URINALYSIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 247
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR URINALYSIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 248
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR URINALYSIS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 249
IN VITRO DIAGNOSTICS MARKET FOR CHROMATOGRAPHY & MASS SPECTROMETRY, BY REGION, 2022–2030 (USD MILLION)
TABLE 250
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CHROMATOGRAPHY & MASS SPECTROMETRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 251
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CHROMATOGRAPHY & MASS SPECTROMETRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 252
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CHROMATOGRAPHY & MASS SPECTROMETRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 253
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CHROMATOGRAPHY & MASS SPECTROMETRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 254
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CHROMATOGRAPHY & MASS SPECTROMETRY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 255
IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 256
KEY BLOOD, SERUM, AND PLASMA SAMPLE TYPE-BASED PRODUCTS AVAILABLE WORLDWIDE
TABLE 257
IN VITRO DIAGNOSTICS MARKET FOR BLOOD, SERUM, AND PLASMA SPECIMENS, BY REGION, 2022–2030 (USD MILLION)
TABLE 258
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD, SERUM, AND PLASMA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 259
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BLOOD, SERUM, AND PLASMA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 260
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BLOOD, SERUM, AND PLASMA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 261
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD, SERUM, AND PLASMA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 262
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BLOOD, SERUM, AND PLASMA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 263
IN VITRO DIAGNOSTICS MARKET FOR SALIVA SPECIMENS, BY REGION, 2022–2030 (USD MILLION)
TABLE 264
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR SALIVA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 265
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR SALIVA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 266
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR SALIVA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 267
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR SALIVA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 268
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR SALIVA SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 269
KEY URINE SAMPLE TYPE-BASED PRODUCTS AVAILABLE WORLDWIDE
TABLE 270
IN VITRO DIAGNOSTICS MARKET FOR URINE SPECIMENS, BY REGION, 2022–2030 (USD MILLION)
TABLE 271
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR URINE SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 272
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR URINE SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 273
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR URINE SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 274
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR URINE SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 275
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR URINE SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 276
IN VITRO DIAGNOSTICS MARKET FOR OTHER SPECIMENS, BY REGION, 2022–2030 (USD MILLION)
TABLE 277
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 278
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR OTHER SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 279
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR OTHER SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 280
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 281
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR OTHER SPECIMENS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 282
IN VITRO DIAGNOSTICS MARKET, BY SITE OF TESTING, 2022–2030 (USD MILLION)
TABLE 283
IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 284
IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY REGION, 2022–2030 (USD MILLION)
TABLE 285
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 286
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 287
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 288
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 289
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 290
IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 291
IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY REGION, 2022–2030 (USD MILLION)
TABLE 292
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 293
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 294
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 295
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 296
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR POINT-OF-CARE TESTS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 297
IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 298
IN VITRO DIAGNOSTICS MARKET FOR INFECTIOUS DISEASES, BY REGION, 2022–2030 (USD MILLION)
TABLE 299
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 300
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 301
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 302
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 303
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 304
INCREASING INCIDENCE OF CANCER, BY REGION, 2022 VS. 2040
TABLE 305
LIST OF APPROVED COMPANION DIAGNOSTIC DEVICES, 2022–2024
TABLE 306
IN VITRO DIAGNOSTICS MARKET FOR ONCOLOGY, BY REGION, 2022–2030 (USD MILLION)
TABLE 307
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 308
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 309
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 310
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 311
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 312
IN VITRO DIAGNOSTICS MARKET FOR ENDOCRINOLOGY, BY REGION, 2022–2030 (USD MILLION)
TABLE 313
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ENDOCRINOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 314
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ENDOCRINOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 315
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ENDOCRINOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 316
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ENDOCRINOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 317
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ENDOCRINOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 318
IN VITRO DIAGNOSTICS MARKET FOR CARDIOLOGY, BY REGION, 2022–2030 (USD MILLION)
TABLE 319
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CARDIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 320
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CARDIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 321
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CARDIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 322
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CARDIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 323
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CARDIOLOGY, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 324
IN VITRO DIAGNOSTICS MARKET FOR BLOOD SCREENING, BY REGION, 2022–2030 (USD MILLION)
TABLE 325
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD SCREENING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 326
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BLOOD SCREENING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 327
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BLOOD SCREENING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 328
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD SCREENING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 329
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BLOOD SCREENING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 330
IN VITRO DIAGNOSTICS MARKET FOR GENETIC TESTING, BY REGION, 2022–2030 (USD MILLION)
TABLE 331
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR GENETIC TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 332
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR GENETIC TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 333
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR GENETIC TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 334
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR GENETIC TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 335
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR GENETIC TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 336
IN VITRO DIAGNOSTICS MARKET FOR AUTOIMMUNE DISEASES, BY REGION, 2022–2030 (USD MILLION)
TABLE 337
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 338
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 339
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 340
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 341
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 342
IN VITRO DIAGNOSTICS MARKET FOR ALLERGY DIAGNOSTICS, BY REGION, 2022–2030 (USD MILLION)
TABLE 343
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ALLERGY DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 344
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ALLERGY DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 345
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ALLERGY DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 346
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ALLERGY DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 347
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ALLERGY DIAGNOSTICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 348
KEY PRODUCTS FOR DRUG MONITORING WORLDWIDE
TABLE 349
KEY IVD PRODUCTS FOR DRUG TESTING WORLDWIDE
TABLE 350
IN VITRO DIAGNOSTICS MARKET FOR DRUG MONITORING & TESTING, BY REGION, 2022–2030 (USD MILLION)
TABLE 351
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DRUG MONITORING & TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 352
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR DRUG MONITORING & TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 353
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR DRUG MONITORING & TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 354
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR DRUG MONITORING & TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 355
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR DRUG MONITORING & TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 356
KEY PRODUCTS FOR BONE & MINERAL DISORDERS WORLDWIDE
TABLE 357
IN VITRO DIAGNOSTICS MARKET FOR BONE & MINERAL DISORDERS, BY REGION, 2022–2030 (USD MILLION)
TABLE 358
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BONE & MINERAL DISORDERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 359
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BONE & MINERAL DISORDERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 360
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BONE & MINERAL DISORDERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 361
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BONE & MINERAL DISORDERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 362
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BONE & MINERAL DISORDERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 363
IN VITRO DIAGNOSTICS MARKET FOR COAGULATION TESTING, BY REGION, 2022–2030 (USD MILLION)
TABLE 364
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 365
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 366
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 367
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 368
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR COAGULATION TESTING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 369
IN VITRO DIAGNOSTICS MARKET FOR BLOOD GROUP TYPING, BY REGION, 2022–2030 (USD MILLION)
TABLE 370
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GROUP TYPING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 371
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GROUP TYPING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 372
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GROUP TYPING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 373
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GROUP TYPING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 374
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BLOOD GROUP TYPING, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 375
IN VITRO DIAGNOSTICS MARKET FOR OTHER APPLICATIONS, BY REGION, 2022–2030 (USD MILLION)
TABLE 376
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 377
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 378
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 379
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 380
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 381
IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 382
IN VITRO DIAGNOSTICS MARKET FOR HOSPITALS & CLINICS, BY REGION, 2022–2030 (USD MILLION)
TABLE 383
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 384
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 385
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 386
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 387
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 388
IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 389
IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY REGION, 2022–2030 (USD MILLION)
TABLE 390
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 391
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 392
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 393
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 394
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 395
IN VITRO DIAGNOSTICS MARKET FOR LARGE REFERENCE LABORATORIES, BY REGION, 2022–2030 (USD MILLION)
TABLE 396
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LARGE REFERENCE LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 397
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR LARGE REFERENCE LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 398
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR LARGE REFERENCE LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 399
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR LARGE REFERENCE LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 400
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR LARGE REFERENCE LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 401
IN VITRO DIAGNOSTICS MARKET FOR SMALL AND MEDIUM-SIZED LABORATORIES, BY REGION, 2022–2030 (USD MILLION)
TABLE 402
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR SMALL AND MEDIUM-SIZED LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 403
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR SMALL AND MEDIUM-SIZED LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 404
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR SMALL AND MEDIUM-SIZED LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 405
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR SMALL AND MEDIUM-SIZED LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 406
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR SMALL AND MEDIUM-SIZED LABORATORIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 407
IN VITRO DIAGNOSTICS MARKET FOR BLOOD BANKS, BY REGION, 2022–2030 (USD MILLION)
TABLE 408
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD BANKS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 409
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR BLOOD BANKS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 410
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR BLOOD BANKS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 411
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR BLOOD BANKS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 412
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR BLOOD BANKS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 413
IN VITRO DIAGNOSTICS MARKET FOR HOME CARE SETTINGS, BY REGION, 2022–2030 (USD MILLION)
TABLE 414
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR HOME CARE SETTINGS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 415
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR HOME CARE SETTINGS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 416
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR HOME CARE SETTINGS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 417
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR HOME CARE SETTINGS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 418
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR HOME CARE SETTINGS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 419
IN VITRO DIAGNOSTICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2022–2030 (USD MILLION)
TABLE 420
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 421
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 422
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 423
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 424
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 425
IN VITRO DIAGNOSTICS MARKET FOR ACADEMIC INSTITUTES, BY REGION, 2022–2030 (USD MILLION)
TABLE 426
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 427
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 428
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 429
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 430
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 431
IN VITRO DIAGNOSTICS MARKET FOR OTHER END USERS, BY REGION, 2022–2030 (USD MILLION)
TABLE 432
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER END USERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 433
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR OTHER END USERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 434
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR OTHER END USERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 435
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR OTHER END USERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 436
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR OTHER END USERS, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 437
IN VITRO DIAGNOSTICS MARKET, BY REGION, 2022–2030 (USD MILLION)
TABLE 438
NORTH AMERICA: KEY MACROECONOMIC INDICATORS
TABLE 439
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 440
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 441
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 442
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 443
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 444
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 445
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 446
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 447
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 448
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 449
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 450
US: KEY MACROECONOMIC INDICATORS
TABLE 451
US: NUMBER OF IN VITRO DIAGNOSTICS TESTS CONDUCTED, 2022–2030 (BILLION)
TABLE 452
US: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 453
US: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 454
US: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 455
US: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 456
US: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 457
US: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 458
US: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 459
US: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 460
US: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 461
US: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 462
CANADA: ESTIMATED PREVALENCE OF DIABETES, 2024 VS. 2034
TABLE 463
CANADA: KEY MACROECONOMIC INDICATORS
TABLE 464
CANADA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 465
CANADA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 466
CANADA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 467
CANADA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 468
CANADA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 469
CANADA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 470
CANADA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 471
CANADA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 472
CANADA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 473
CANADA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 474
EUROPE: KEY MACROECONOMIC INDICATORS
TABLE 475
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 476
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 477
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 478
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 479
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 480
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 481
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 482
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 483
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 484
EUROPE: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 485
EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 486
GERMANY: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 487
GERMANY: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 488
GERMANY: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 489
GERMANY: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 490
GERMANY: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 491
GERMANY: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 492
GERMANY: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 493
GERMANY: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 494
GERMANY: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 495
GERMANY: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 496
FRANCE: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 497
FRANCE: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 498
FRANCE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 499
FRANCE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 500
FRANCE: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 501
FRANCE: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 502
FRANCE: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 503
FRANCE: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 504
FRANCE: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 505
FRANCE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 506
UK: KEY MACROECONOMIC INDICATORS
TABLE 507
UK: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 508
UK: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 509
UK: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 510
UK: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 511
UK: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 512
UK: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 513
UK: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 514
UK: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 515
UK: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 516
UK: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 517
ITALY: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 518
ITALY: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 519
ITALY: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 520
ITALY: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 521
ITALY: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 522
ITALY: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 523
ITALY: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 524
ITALY: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 525
ITALY: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 526
ITALY: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 527
SPAIN: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 528
SPAIN: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 529
SPAIN: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 530
SPAIN: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 531
SPAIN: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 532
SPAIN: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 533
SPAIN: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 534
SPAIN: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 535
SPAIN: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 536
SPAIN: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 537
RUSSIA: KEY MACROECONOMIC INDICATORS
TABLE 538
RUSSIA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 539
RUSSIA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 540
RUSSIA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 541
RUSSIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 542
RUSSIA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 543
RUSSIA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 544
RUSSIA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 545
RUSSIA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 546
RUSSIA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 547
RUSSIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 548
SWITZERLAND: KEY MACROECONOMIC INDICATORS
TABLE 549
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 550
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 551
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 552
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 553
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 554
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 555
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 556
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 557
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 558
SWITZERLAND: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 559
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 560
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 561
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 562
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 563
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 564
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 565
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 566
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 567
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 568
REST OF EUROPE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 569
ASIA PACIFIC: KEY MACROECONOMIC INDICATORS
TABLE 570
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 571
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 572
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 573
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 574
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 575
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 576
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 577
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 578
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 579
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 580
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 581
JAPAN: KEY MACROECONOMIC INDICATORS
TABLE 582
JAPAN: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 583
JAPAN: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 584
JAPAN: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 585
JAPAN: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 586
JAPAN: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 587
JAPAN: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 588
JAPAN: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 589
JAPAN: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 590
JAPAN: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 591
JAPAN: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 592
CHINA: KEY MACROECONOMIC INDICATORS
TABLE 593
CHINA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 594
CHINA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 595
CHINA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 596
CHINA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 597
CHINA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 598
CHINA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 599
CHINA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 600
CHINA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 601
CHINA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 602
CHINA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 603
INDIA: KEY MACROECONOMIC INDICATORS
TABLE 604
INDIA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 605
INDIA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 606
INDIA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 607
INDIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 608
INDIA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 609
INDIA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 610
INDIA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 611
INDIA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 612
INDIA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 613
INDIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 614
SOUTH KOREA: KEY MACROECONOMIC INDICATORS
TABLE 615
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 616
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 617
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 618
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 619
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 620
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 621
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 622
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 623
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 624
SOUTH KOREA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 625
AUSTRALIA: KEY MACROECONOMIC INDICATORS
TABLE 626
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 627
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 628
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 629
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 630
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 631
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 632
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 633
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 634
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 635
AUSTRALIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 636
SINGAPORE: KEY MACROECONOMIC INDICATORS
TABLE 637
SINGAPORE: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 638
SINGAPORE: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 639
SINGAPORE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 640
SINGAPORE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 641
SINGAPORE: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 642
SINGAPORE: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 643
SINGAPORE: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 644
SINGAPORE: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 645
SINGAPORE: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 646
SINGAPORE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 647
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 648
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 649
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 650
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 651
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 652
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 653
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 654
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 655
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 656
REST OF ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 657
LATIN AMERICA: KEY MACROECONOMIC INDICATORS
TABLE 658
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 659
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 660
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 661
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 662
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 663
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 664
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 665
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 666
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 667
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 668
LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 669
BRAZIL: KEY MACROECONOMIC INDICATORS
TABLE 670
BRAZIL: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 671
BRAZIL: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 672
BRAZIL: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 673
BRAZIL: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 674
BRAZIL: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 675
BRAZIL: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 676
BRAZIL: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 677
BRAZIL: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 678
BRAZIL: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 679
BRAZIL: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 680
MEXICO: KEY MACROECONOMIC INDICATORS
TABLE 681
MEXICO: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 682
MEXICO: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 683
MEXICO: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 684
MEXICO: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 685
MEXICO: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 686
MEXICO: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 687
MEXICO: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 688
MEXICO: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 689
MEXICO: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 690
MEXICO: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 691
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 692
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 693
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 694
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 695
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 696
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 697
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 698
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 699
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 700
REST OF LATIN AMERICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 701
MIDDLE EAST & AFRICA: KEY MACROECONOMIC INDICATORS
TABLE 702
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 703
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 704
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 705
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 706
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 707
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 708
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 709
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 710
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 711
MIDDLE EAST & AFRICA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 712
GCC COUNTRIES: KEY MACROECONOMIC INDICATORS
TABLE 713
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY COUNTRY, 2022–2030 (USD MILLION)
TABLE 714
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 715
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 716
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 717
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 718
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 719
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 720
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 721
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 722
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 723
GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 724
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 725
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 726
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 727
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 728
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 729
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 730
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 731
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 732
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 733
SAUDI ARABIA: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 734
UAE: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 735
UAE: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 736
UAE: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 737
UAE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 738
UAE: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 739
UAE: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 740
UAE: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 741
UAE: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 742
UAE: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 743
UAE: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 744
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2022–2030 (USD MILLION)
TABLE 745
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2022–2030 (USD MILLION)
TABLE 746
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR IMMUNOASSAYS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 747
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL CHEMISTRY, BY TYPE, 2022–2030 (USD MILLION)
TABLE 748
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR MOLECULAR DIAGNOSTICS, BY TYPE, 2022–2030 (USD MILLION)
TABLE 749
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2022–2030 (USD MILLION)
TABLE 750
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY TEST TYPE, 2022–2030 (USD MILLION)
TABLE 751
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2022–2030 (USD MILLION)
TABLE 752
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET, BY END USER, 2022–2030 (USD MILLION)
TABLE 753
OTHER GCC COUNTRIES: IN VITRO DIAGNOSTICS MARKET FOR CLINICAL LABORATORIES, BY TYPE, 2022–2030 (USD MILLION)
TABLE 754
OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS, JANUARY 2022–APRIL 2025
TABLE 755
IN VITRO DIAGNOSTICS MARKET: DEGREE OF COMPETITION
TABLE 756
IN VITRO DIAGNOSTICS MARKET: REGION FOOTPRINT
TABLE 757
IN VITRO DIAGNOSTICS MARKET: PRODUCT & SERVICE FOOTPRINT
TABLE 758
IN VITRO DIAGNOSTICS MARKET: TECHNOLOGY FOOTPRINT
TABLE 759
IN VITRO DIAGNOSTICS MARKET: END-USER FOOTPRINT
TABLE 760
IN VITRO DIAGNOSTICS MARKET: DETAILED LIST OF KEY STARTUPS/SMES
TABLE 761
IN VITRO DIAGNOSTICS MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES
TABLE 762
IN VITRO DIAGNOSTICS MARKET: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–APRIL 2025
TABLE 763
IN VITRO DIAGNOSTICS MARKET: DEALS, JANUARY 2022–APRIL 2025
TABLE 764
IN VITRO DIAGNOSTICS MARKET: EXPANSIONS, JANUARY 2022–APRIL 2025
TABLE 765
IN VITRO DIAGNOSTICS MARKET: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 766
IN VITRO DIAGNOSTICS MARKET: DEALS, JANUARY 2022–MARCH 2025
TABLE 767
AI-DRIVEN PRODUCTS & SERVICES PROVIDED BY KEY PLAYERS
TABLE 768
DANAHER: COMPANY OVERVIEW
TABLE 769
DANAHER: PRODUCTS/SERVICES OFFERED
TABLE 770
DANAHER: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 771
DANAHER: DEALS, JANUARY 2022–MARCH 2025
TABLE 772
DANAHER: EXPANSIONS, JANUARY 2022–MARCH 2024
TABLE 773
F. HOFFMANN-LA ROCHE LTD: COMPANY OVERVIEW
TABLE 774
F. HOFFMANN-LA ROCHE LTD: CURRENCY CONVERSION, 2022–2024
TABLE 775
F. HOFFMANN-LA ROCHE LTD: PRODUCTS/SERVICES OFFERED
TABLE 776
F. HOFFMANN-LA ROCHE LTD: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 777
F. HOFFMANN-LA ROCHE LTD: DEALS, JANUARY 2022–MARCH 2025
TABLE 778
F. HOFFMANN-LA ROCHE LTD: EXPANSIONS, JANUARY 2022–MARCH 2024
TABLE 779
ABBOTT: COMPANY OVERVIEW
TABLE 780
ABBOTT: PRODUCTS/SERVICES OFFERED
TABLE 781
ABBOTT: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 782
ABBOTT: DEALS, JANUARY 2022–MARCH 2025
TABLE 783
ABBOTT: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 784
SIEMENS HEALTHINEERS AG: COMPANY OVERVIEW
TABLE 785
SIEMENS HEALTHINEERS AG: CURRENCY CONVERSION, 2022–2024
TABLE 786
SIEMENS HEALTHINEERS AG: PRODUCTS/SERVICES OFFERED
TABLE 787
SIEMENS HEALTHINEERS AG: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 788
SIEMENS HEALTHINEERS AG: DEALS, JANUARY 2022–MARCH 2025
TABLE 789
SIEMENS HEALTHINEERS AG: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 790
THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
TABLE 791
THERMO FISHER SCIENTIFIC INC.: PRODUCTS/SERVICES OFFERED
TABLE 792
THERMO FISHER SCIENTIFIC INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 793
THERMO FISHER SCIENTIFIC INC.: DEALS, JANUARY 2022–MARCH 2025
TABLE 794
THERMO FISHER SCIENTIFIC INC.: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 795
ILLUMINA, INC.: COMPANY OVERVIEW
TABLE 796
ILLUMINA, INC.: PRODUCTS OFFERED
TABLE 797
ILLUMINA, INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 798
ILLUMINA, INC.: DEALS, JANUARY 2022–MARCH 2025
TABLE 799
ILLUMINA, INC.: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 800
ILLUMINA, INC.: OTHER DEVELOPMENTS, JANUARY 2022–MARCH 2025
TABLE 801
HOLOGIC, INC.: COMPANY OVERVIEW
TABLE 802
HOLOGIC, INC.: PRODUCTS/SERVICES OFFERED
TABLE 803
HOLOGIC, INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 804
HOLOGIC, INC.: DEALS, JANUARY 2022–MARCH 2025
TABLE 805
HOLOGIC, INC.: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 806
BIO-RAD LABORATORIES, INC.: COMPANY OVERVIEW
TABLE 807
BIO-RAD LABORATORIES, INC.: PRODUCTS/SERVICES OFFERED
TABLE 808
BIO-RAD LABORATORIES, INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 809
BIO-RAD LABORATORIES, INC.: DEALS, JANUARY 2022–MARCH 2025
TABLE 810
BIOMÉRIEUX: COMPANY OVERVIEW
TABLE 811
BIOMÉRIEUX: CURRENCY CONVERSION, 2022–2024
TABLE 812
BIOMÉRIEUX: PRODUCTS/SERVICES OFFERED
TABLE 813
BIOMÉRIEUX: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 814
BIOMÉRIEUX: DEALS, JANUARY 2022–MARCH 2025
TABLE 815
BIOMÉRIEUX: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 816
SYSMEX CORPORATION: COMPANY OVERVIEW
TABLE 817
SYSMEX CORPORATION: CURRENCY CONVERSION, 2022–2024
TABLE 818
SYSMEX CORPORATION: PRODUCTS/SERVICES OFFERED
TABLE 819
SYSMEX CORPORATION: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 820
SYSMEX CORPORATION: DEALS, JANUARY 2022–MARCH 2025
TABLE 821
SYSMEX CORPORATION: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 822
REVVITY: COMPANY OVERVIEW
TABLE 823
REVVITY: PRODUCTS/SERVICES OFFERED
TABLE 824
REVVITY: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 825
REVVITY: DEALS, JANUARY 2022–MARCH 2025
TABLE 826
REVVITY: OTHER DEVELOPMENTS, JANUARY 2022–MARCH 2025
TABLE 827
BECTON, DICKINSON AND COMPANY: COMPANY OVERVIEW
TABLE 828
BECTON, DICKINSON AND COMPANY: PRODUCTS/SERVICES OFFERED
TABLE 829
BECTON, DICKINSON AND COMPANY: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 830
BECTON, DICKINSON AND COMPANY: DEALS, JANUARY 2022–MARCH 2025
TABLE 831
BECTON, DICKINSON AND COMPANY: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 832
AGILENT TECHNOLOGIES, INC.: COMPANY OVERVIEW
TABLE 833
AGILENT TECHNOLOGIES, INC: PRODUCTS/SERVICES OFFERED
TABLE 834
AGILENT TECHNOLOGIES, INC: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 835
AGILENT TECHNOLOGIES, INC: DEALS, JANUARY 2022–MARCH 2025
TABLE 836
AGILENT TECHNOLOGIES, INC: OTHER DEVELOPMENTS, JANUARY 2022–MARCH 2025
TABLE 837
QIAGEN: COMPANY OVERVIEW
TABLE 838
QIAGEN: PRODUCTS/SERVICES OFFERED
TABLE 839
QIAGEN: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 840
QIAGEN: DEALS, JANUARY 2022–MARCH 2025
TABLE 841
DIASORIN S.P.A.: COMPANY OVERVIEW
TABLE 842
DIASORIN S.P.A.: CURRENCY CONVERSION, 2021-2023
TABLE 843
DIASORIN S.P.A.: PRODUCTS/SERVICES OFFERED
TABLE 844
DIASORIN S.P.A.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 845
DIASORIN S.P.A.: DEALS, JANUARY 2022–MARCH 2025
TABLE 846
GRIFOLS, S.A.: COMPANY OVERVIEW
TABLE 847
GRIFOLS, S.A.: CURRENCY CONVERSION, 2022–2024
TABLE 848
GRIFOLS, S.A.: PRODUCTS/SERVICES OFFERED
TABLE 849
GRIFOLS, S.A.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 850
GRIFOLS, S.A.: DEALS, JANUARY 2022–MARCH 2025
TABLE 851
WERFEN: COMPANY OVERVIEW
TABLE 852
WERFEN: PRODUCTS/SERVICES OFFERED
TABLE 853
WERFEN: DEALS, JANUARY 2022–MARCH 2025
TABLE 854
WERFEN: EXPANSIONS, JANUARY 2022–MARCH 2025
TABLE 855
QUIDELORTHO CORPORATION: COMPANY OVERVIEW
TABLE 856
QUIDELORTHO CORPORATION: PRODUCTS/SERVICES OFFERED
TABLE 857
QUIDELORTHO CORPORATION: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2022–MARCH 2025
TABLE 858
QUIDELORTHO CORPORATION: DEALS, JANUARY 2022–MARCH 2025
TABLE 859
QUIDELORTHO CORPORATION: EXPANSIONS, JANUARY 2022–MARCH 2024
TABLE 860
DEVYSER: COMPANY OVERVIEW
TABLE 861
BIOSYNEX SA: COMPANY OVERVIEW
TABLE 862
SURMODICS, INC.: COMPANY OVERVIEW
TABLE 863
MENARINI SILICON BIOSYSTEMS: COMPANY OVERVIEW
TABLE 864
SPEEDX PTY. LTD.: COMPANY OVERVIEW
TABLE 865
GENSPEED BIOTECH GMBH: COMPANY OVERVIEW
TABLE 866
MERCK KGAA: COMPANY OVERVIEW
TABLE 867
CARIS LIFE SCIENCES: COMPANY OVERVIEW
TABLE 868
ARKRAY, INC.: COMPANY OVERVIEW
TABLE 869
ACCELERATE DIAGNOSTICS, INC.: COMPANY OVERVIEW
TABLE 870
CELLABS: COMPANY OVERVIEW
TABLE 871
J. MITRA & CO. PVT. LTD.: COMPANY OVERVIEW
TABLE 872
EPITOPE DIAGNOSTICS, INC.: COMPANY OVERVIEW
TABLE 873
BOSTER BIOLOGICAL TECHNOLOGY: COMPANY OVERVIEW
TABLE 874
ENZO BIOCHEM INC.: COMPANY OVERVIEW
TABLE 875
GENETIC SIGNATURES: COMPANY OVERVIEW
TABLE 876
SAVYON DIAGNOSTICS: COMPANY OVERVIEW
TABLE 877
TRIVITRON HEALTHCARE: COMPANY OVERVIEW
TABLE 878
MDXHEALTH: COMPANY OVERVIEW
TABLE 879
CREATIVE DIAGNOSTICS: COMPANY OVERVIEW
TABLE 880
INBIOS INTERNATIONAL, INC.: COMPANY OVERVIEW
TABLE 881
MACCURA BIOTECHNOLOGY CO., LTD.: COMPANY OVERVIEW
TABLE 882
LUYE LIFE SCIENCES GROUP: COMPANY OVERVIEW
FIGURE 1
IN VITRO DIAGNOSTICS MARKET SEGMENTATION AND REGIONAL SCOPE
FIGURE 3
BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY-SIDE AND DEMAND-SIDE PARTICIPANTS
FIGURE 4
BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
FIGURE 5
GLOBAL IN VITRO DIAGNOSTICS MARKET: REVENUE SHARE ANALYSIS
FIGURE 6
CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
FIGURE 7
TOP-DOWN APPROACH
FIGURE 8
DATA TRIANGULATION METHODOLOGY
FIGURE 9
IN VITRO DIAGNOSTICS MARKET, BY PRODUCT & SERVICE, 2025 VS. 2030 (USD MILLION)
FIGURE 10
IN VITRO DIAGNOSTICS MARKET, BY TECHNOLOGY, 2025 VS. 2030 (USD MILLION)
FIGURE 11
IN VITRO DIAGNOSTICS MARKET, BY SPECIMEN, 2025 VS. 2030 (USD MILLION)
FIGURE 12
IN VITRO DIAGNOSTICS MARKET, BY SITE OF TESTING, 2025 VS. 2030 (USD MILLION)
FIGURE 13
IN VITRO DIAGNOSTICS MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
FIGURE 14
IN VITRO DIAGNOSTICS MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
FIGURE 15
GEOGRAPHIC SNAPSHOT OF IN VITRO DIAGNOSTICS MARKET
FIGURE 16
GROWING PREVALENCE OF INFECTIOUS DISEASES AND RISING EMPHASIS ON NEED FOR EARLY DIAGNOSIS TO DRIVE MARKET
FIGURE 17
REAGENTS & KITS SEGMENT AND US LED NORTH AMERICAN IN VITRO DIAGNOSTICS MARKET IN 2024
FIGURE 18
CHINA TO REGISTER HIGHEST CAGR DURING FORECAST PERIOD
FIGURE 19
ASIA PACIFIC TO WITNESS HIGHEST GROWTH DURING FORECAST PERIOD
FIGURE 20
EMERGING ECONOMIES TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
FIGURE 21
IN VITRO DIAGNOSTICS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
FIGURE 22
IN VITRO DIAGNOSTIC REGULATION: CRITICAL AREAS OF IMPACT
FIGURE 23
TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
FIGURE 24
IN VITRO DIAGNOSTICS MARKET: VALUE CHAIN ANALYSIS
FIGURE 25
IN VITRO DIAGNOSTICS MARKET: SUPPLY CHAIN ANALYSIS
FIGURE 26
IN VITRO DIAGNOSTICS MARKET: ECOSYSTEM MAPPING
FIGURE 27
IN VITRO DIAGNOSTICS MARKET: INVESTMENT AND FUNDING SCENARIO, 2020−2023
FIGURE 28
IN VITRO DIAGNOSTICS MARKET: PATENT ANALYSIS, 2014–2024
FIGURE 29
US: REGULATORY PROCESS FOR IVD DEVICES
FIGURE 30
CANADA: REGULATORY PROCESS FOR IVD DEVICES IN CANADA
FIGURE 31
EUROPE: IN VITRO DIAGNOSTIC REGULATION (IVDR) TIMELINE
FIGURE 32
REGULATORY PROCESS FOR IVD DEVICES IN JAPAN
FIGURE 33
INDIA: REGULATORY PROCESS FOR IVD DEVICES
FIGURE 34
MEXICO: REGULATORY PROCESS FOR IVD DEVICES
FIGURE 35
IN VITRO DIAGNOSTICS MARKET: PORTER’S FIVE FORCES ANALYSIS
FIGURE 36
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF IVD PRODUCTS, BY PRODUCT & SERVICE
FIGURE 37
KEY BUYING CRITERIA, BY PRODUCT & SERVICE
FIGURE 39
NORTH AMERICA: IN VITRO DIAGNOSTICS MARKET SNAPSHOT
FIGURE 40
ASIA PACIFIC: IN VITRO DIAGNOSTICS MARKET SNAPSHOT
FIGURE 41
IN VITRO DIAGNOSTICS MARKET: REVENUE ANALYSIS OF KEY PLAYERS, 2022–2024 (USD BILLION)
FIGURE 42
IN VITRO DIAGNOSTICS MARKET SHARE ANALYSIS, 2024
FIGURE 43
RANKING OF KEY PLAYERS, 2024
FIGURE 44
EV/EBITDA OF KEY VENDORS, 2025
FIGURE 45
YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS, 2025
FIGURE 46
IN VITRO DIAGNOSTICS MARKET: BRAND/PRODUCT COMPARISON
FIGURE 47
IN VITRO DIAGNOSTICS MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
FIGURE 48
IN VITRO DIAGNOSTICS MARKET: COMPANY FOOTPRINT
FIGURE 49
IN VITRO DIAGNOSTICS MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
FIGURE 50
DANAHER: COMPANY SNAPSHOT (2024)
FIGURE 51
F. HOFFMANN-LA ROCHE LTD: COMPANY SNAPSHOT (2024)
FIGURE 52
ABBOTT: COMPANY SNAPSHOT (2024)
FIGURE 53
SIEMENS HEALTHINEERS AG: COMPANY SNAPSHOT (2024)
FIGURE 54
THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2024)
FIGURE 55
ILLUMINA, INC.: COMPANY SNAPSHOT (2024)
FIGURE 56
HOLOGIC, INC.: COMPANY SNAPSHOT (2024)
FIGURE 57
BIO-RAD LABORATORIES, INC.: COMPANY SNAPSHOT (2024)
FIGURE 58
BIOMÉRIEUX: COMPANY SNAPSHOT (2024)
FIGURE 59
SYSMEX CORPORATION: COMPANY SNAPSHOT (2024)
FIGURE 60
REVVITY: COMPANY SNAPSHOT (2024)
FIGURE 61
BECTON, DICKINSON AND COMPANY: COMPANY SNAPSHOT (2024)
FIGURE 62
AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT (2024)
FIGURE 63
QIAGEN: COMPANY SNAPSHOT (2024)
FIGURE 64
DIASORIN S.P.A: COMPANY SNAPSHOT (2023)
FIGURE 65
GRIFOLS, S.A.: COMPANY SNAPSHOT (2024)
FIGURE 66
WERFEN: COMPANY SNAPSHOT (2024)
FIGURE 67
QUIDELORTHO CORPORATION: COMPANY SNAPSHOT (2024)





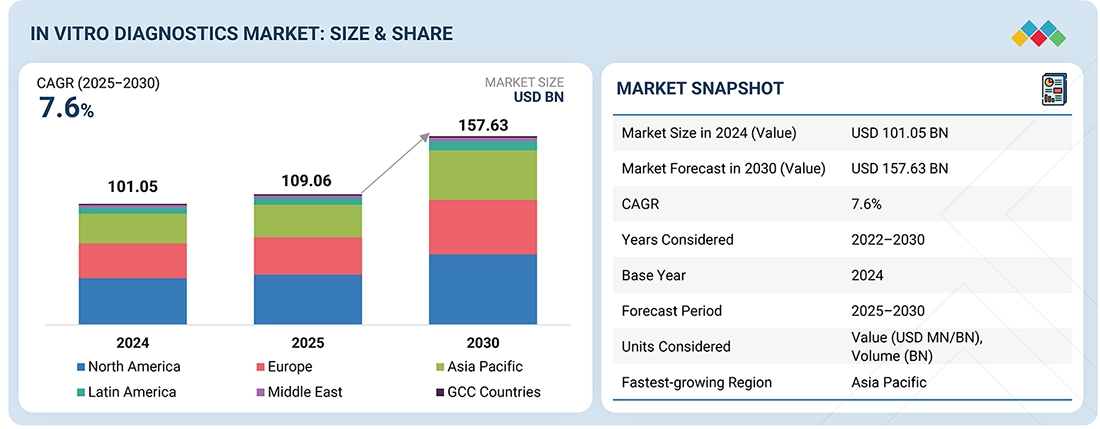
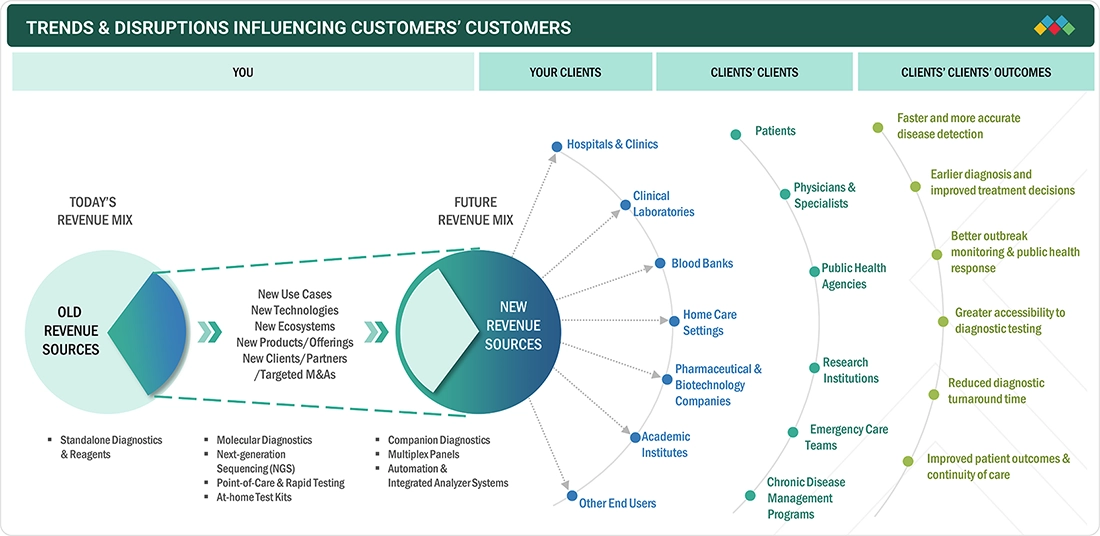







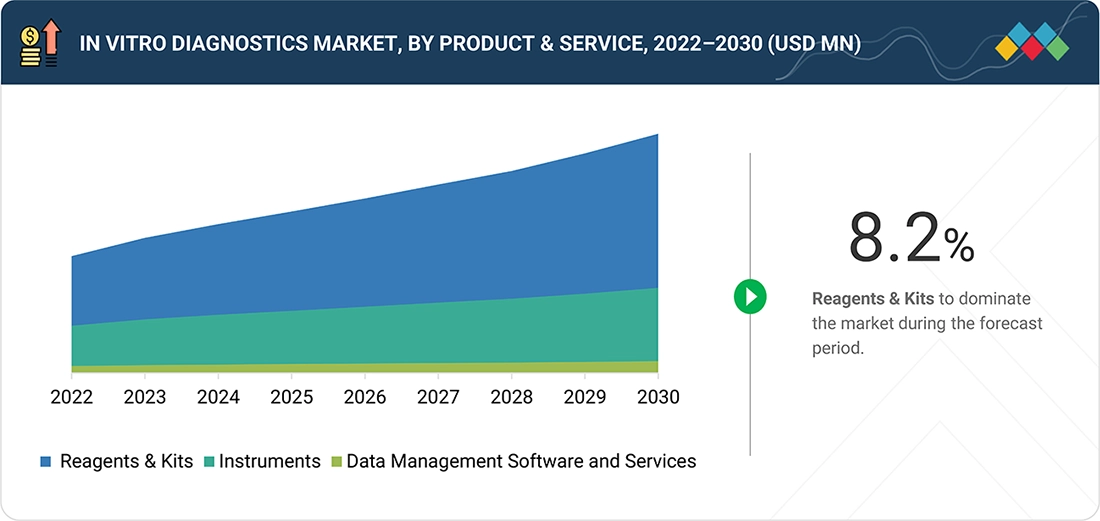
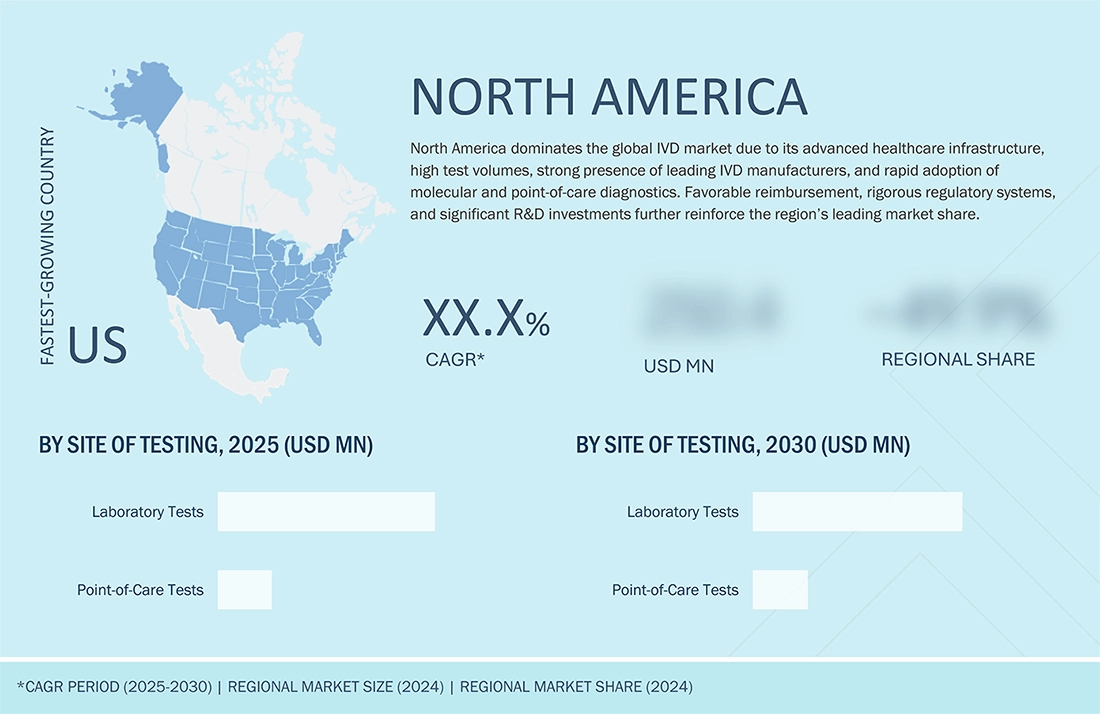
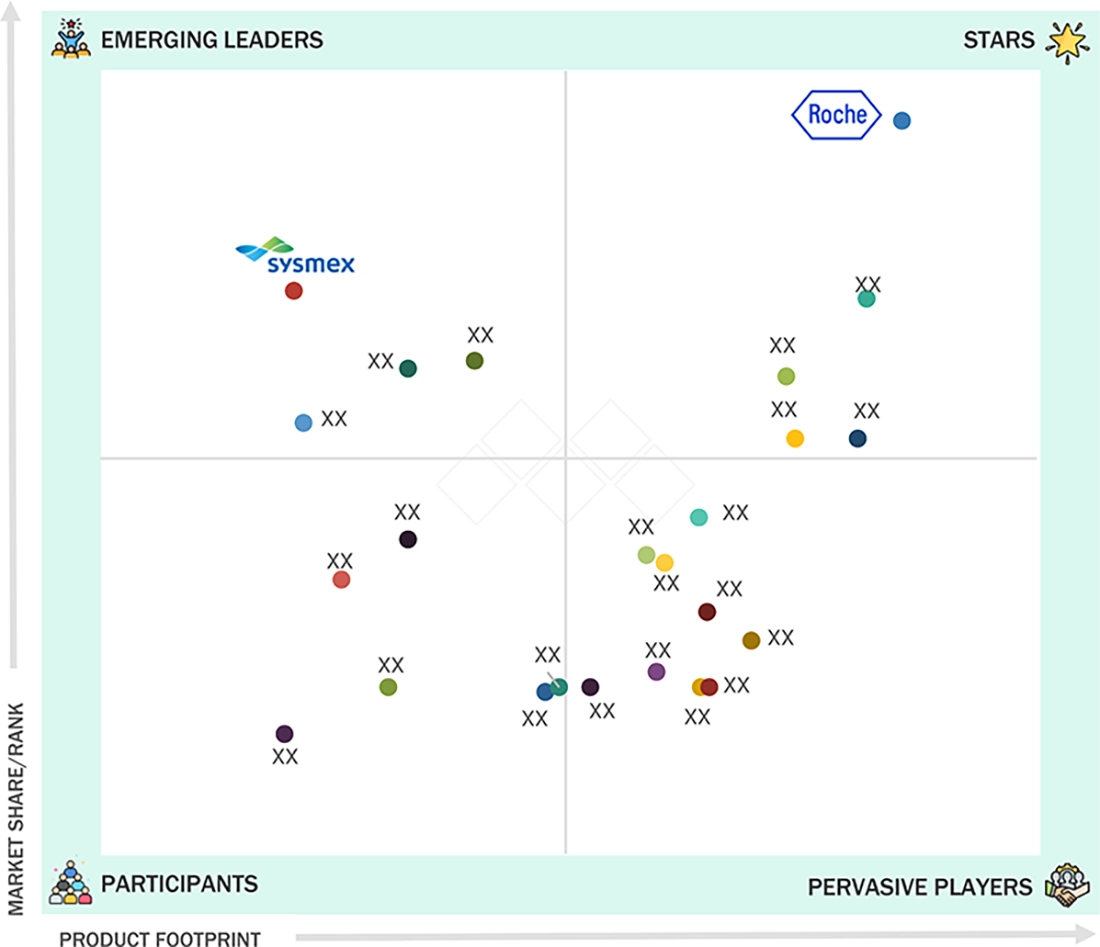


















John
Jun, 2022
Who are the key companies/players in the in-vitro diagnostics market?.
James
Jun, 2022
What are some of the notable developments in the IVD market in recent years?.
Kahlill
Jun, 2022
What are the upcoming trends of In Vitro Diagnostics Market in the world?.