Stem Cell Assays Market Size by Type (Viability, Proliferation, Differentiation, Apoptosis), Cell Type (Mesenchymal, iPSCs, HSCs, hESCs), Product & Service (Instrument), Application (Regenerative Medicine, Clinical Research), End User - Global Forecast to 2027
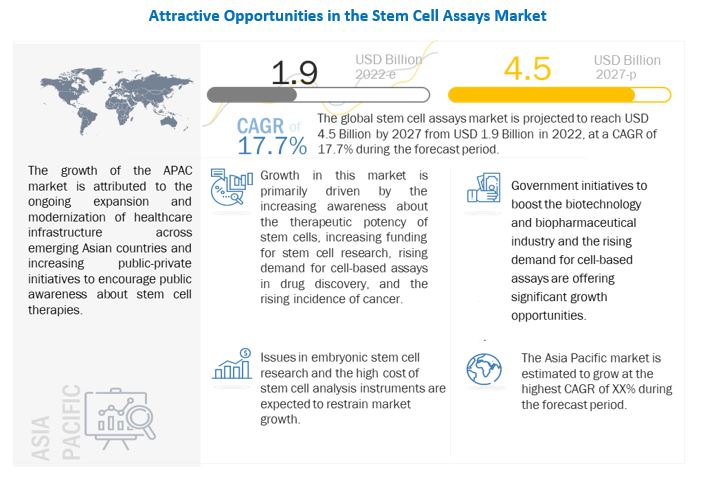
The size of global stem cell assays market in terms of revenue was estimated to be worth USD 1.9 billion in 2022 and is poised to reach USD 4.5 billion by 2027, growing at a CAGR of 17.7% from 2022 to 2027. The research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying behaviour in the market.
Growth in the stem cell assays market can be attributed to factors such as the increasing awareness about the therapeutic potency of stem cells, increasing funding for stem cell research, rising demand for cell-based assays in drug discovery, collaborations and agreements among market players.
To know about the assumptions considered for the study, Request for Free Sample Report
Global Stem Cell Assays Market Dynamics
Driver: Increasing funding for stem cell research
In recent years, companies working on stem cell therapies have received venture capital investments. This has boosted the development of new therapies for diseases such as cancer. For instance, in March 2022, the City of Hope’s Irell & Manella Graduate School of Biological Sciences at Beckman Research Institute received a USD 4.9 million grant from the California Institute for Regenerative Medicine. The funding will help train the scientific community in stem cell research and its translation into novel lifesaving treatments.
Restraint: Issues in embryonic stem cell research
The study of embryonic stem cells allows researchers to understand the development of the human body under normal conditions and how these cells are derived and function. These aspects are essential for developing treatments in regenerative medicine. However, there are a number of ethical issues related to the use of stem cells. Human embryonic stem cells are generated from destroying preimplantation-stage human embryos. This is an ethical issue, as many religions strictly prohibit the destruction of human embryos for research purposes.
Opportunity: Government initiatives to boost stem cell research
Government bodies across various countries have undertaken initiatives to boost their respective biotechnology and biopharmaceutical industries. This is projected to offer growth opportunities in the market. For instance, in February 2022, the Government of India announced the establishment of state-of-the-art stem cell research facilities in 40 research and educational institutions. In the last three years, the government has spent around USD 1 million through the Indian Council of Medical Research (ICMR) for stem cell research.
Challenge: Dearth of trained and skilled professionals
The proper use of flow cytometers, cell counters, and cell imaging and analysis instruments for stem cell analysis requires expertise with relevant experience and knowledge of the technology. The absence of such expertise among stem cell researchers and scientists is limiting the adoption of advanced instruments.
The viability/cytotoxicity assays segment is expected to have the dominant share of the stem cell assays industry in 2021
On the basis of type, the stem cell assays market is segmented into viability/cytotoxicity, isolation & purification, cell identification, proliferation, differentiation, function, and apoptosis assays. The viability/cytotoxicity assays segment is further categorized into tetrazolium reduction assays, resazurin cell viability assays, calcein-AM cell viability assays, and other viability/cytotoxicity assays. The apoptosis assays segment is further categorized into caspase assays, annexin v and cell permeability assays, DNA fragmentation assays, and mitochondrial assays. The viability/cytotoxicity assays segment accounted for the largest share of this market in 2021. Growth fo the segment is attributed to the increased adoption of assays in toxicology & pharmacology.
North America was the largest regional market for Stem cell assays market in 2021.
The market is segmented into five major regions—North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2021, North America accounted for the largest share of the stem cell assays market, owing to the robust research infrastructure in the region and the availability of funding and grants to support the clinical research on stem cells.
To know about the assumptions considered for the study, download the pdf brochure
Key players in the market include Thermo Fisher Scientific Inc. (US), Merck KGaA (Germany), Danaher (US), Becton, Dickinson and Company (US), Bio-Rad Laboratories (US), Promega Corporation (US), Bio-Techne Corporation (US), among others.
Scope of the Stem Cell Assays Industry
|
Report Metric |
Details |
|
Market Revenue Size in 2022 |
$1.9 billion |
|
Projected Revenue Size by 2027 |
$4.5 billion |
|
Industry Growth Rate |
Poised to Grow at a CAGR of 17.7% |
|
Market Driver |
Increasing funding for stem cell research |
|
Market Opportunity |
Government initiatives to boost stem cell research |
This report categorizes the stem cell assays market to forecast revenue and analyze trends in each of the following submarkets:
By Type
- Viability/Cytotoxicity Assays
- Tetrazolium Reduction Assays
- Resazurin Cell Viability Assays
- Calcein-AM Cell Viability Assays
- Other Viability/Cytotoxicity Assays
- Isolation & Purification Assays
- Cell Identification Assays
- Proliferation Assays
- Differentiation Assays
- Function Assays
- Apoptosis Assays
- Caspase Assays
- Annexin V and Cell Permeability Assays
- DNA Fragmentation Assays
- Mitochondrial Assays
By Cell Type
- Adult Stem Cells
- Mesenchymal Stem Cells
- Induced Pluripotent Stem Cells
- Hematopoietic Stem Cells
- Umbilical Cord Stem Cells
- Neural Stem Cells
- Human Embryonic Stems Cells
By Product & Service
- Instruments
- Flow Cytometers
- Microelectrode Arrays
- Cell Imaging & Analysis Systems
- Automated Cell Counters
- Kits
- Services
By Application
- Regenerative Medicine & Therapy Development
- Orthopedic, Musculoskeletal, and Spine Applications
- Dermatology Applications
- Cardiovascular Applications
- CNS Applications
- Oncology Applications
- Diabetes Applications
- Other Regenerative Medicine & Therapy Development Applications
- Drug Discovery & Development
- Clinical Research
By End User
- Biopharmaceutical & Biotechnology Companies
- Academic & Research Institutes
By Region
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Italy
- Spain
- Switzerland
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
Recent Developments of Stem Cell Assays Industry:
- In January 2022, Agilent Technologies Inc. launched Seahorse XF Pro Analyzer, enabling operators at any skill level to access the most advanced cellular metabolism analysis technology for understanding cellular fate, fitness, and function.
- In April 2020, Bio-Rad Laboratories acquired Celsee, Inc., a company that offers instruments and consumables for the isolation, detection, and analysis of single cells that have applications in stem cell analysis. With this acquisition, the company aims to expand its presence in the single-cell analysis market.
Frequently Asked Questions (FAQ):
What is the impact of COVID-19 on the stem cell assays market?
The COVID-19 pandemic has increased the burden on healthcare systems across the globe. According to the World Health Organization (WHO), there were 530,469,195 confirmed cases of COVID-19 and 530,469,195 deaths as of May 27, 2022. Due to the pandemic, cancer and stem cell research funding was affected by the pandemic. Some cancer research institutes have reduced their funding for cancer research in response to the pandemic, while some are still investing in the area. Prominent players operating in the market are altering their long-term and short-term growth strategies by tapping into opportunities prevalent in the research market by developing innovative products to combat the pandemic. This has led to a positive impact on the growth of the market
Who are the key players in the stem cell assays market?
Key players in the Market include Thermo Fisher Scientific Inc. (US), Merck KGaA (Germany), Danaher (US), Becton, Dickinson and Company (US), Bio-Rad Laboratories (US), Promega Corporation (US), and Bio-Techne Corporation (US).
Which type segment dominates the stem cell assays market?
The viability/cytotoxicity segment will continue to dominated the market in the type segment due to the increased adoption of assays in toxicology & pharmacology.
Which application segment dominates the stem cell assays market?
The regenerative medicine & therapy development segment accounted for the largest share of the stem cell assays market, by application, in 2021 owing to its increased usage of stem cell assays in regenerative medicine.
What is the market size for the stem cell assays market?
The stem cell assays market is projected to reach USD 4.5 Billion by 2027 from USD 1.9 Billion in 2022, at a CAGR of 17.7% during the forecast period.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst

TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 31)
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.2.1 INCLUSIONS & EXCLUSIONS
1.3 MARKET SCOPE
1.3.1 MARKETS COVERED
1.3.2 YEARS CONSIDERED FOR THE STUDY
1.4 CURRENCY
1.5 LIMITATIONS
1.6 STAKEHOLDERS
1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY (Page No. - 37)
2.1 RESEARCH DATA
2.1.1 SECONDARY DATA
2.1.2 PRIMARY DATA
2.2 MARKET ESTIMATION METHODOLOGY
2.2.1 INSIGHTS FROM PRIMARY EXPERTS
2.3 MARKET GROWTH RATE PROJECTIONS
2.3.1 DATA TRIANGULATION
2.4 RESEARCH ASSUMPTIONS
2.4.1 COVID-19 ASSUMPTIONS
2.5 RISK ANALYSIS
3 EXECUTIVE SUMMARY (Page No. - 49)
4 PREMIUM INSIGHTS (Page No. - 54)
4.1 STEM CELL ASSAYS MARKET OVERVIEW
4.2 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE AND COUNTRY (2021)
4.3 STEM CELL ASSAYS MARKET SHARE, BY TYPE, 2022 VS. 2027
4.4 STEM CELL ASSAYS MARKET SHARE, BY APPLICATION, 2021
4.5 STEM CELL ASSAYS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW (Page No. - 58)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
5.2.1 DRIVERS
5.2.1.1 Increasing awareness about therapeutic potency of stem cells
5.2.1.2 Increasing funding for stem cell research
5.2.1.3 Rising demand for cell-based assays in drug discovery
5.2.1.4 Collaborations and agreements among market players for stem cell assay products & services
5.2.1.5 Rising incidence of cancer
5.2.2 RESTRAINTS
5.2.2.1 Issues in embryonic stem cell research
5.2.2.2 High cost of stem cell analysis instruments
5.2.3 OPPORTUNITIES
5.2.3.1 Emerging economies
5.2.3.2 Government initiatives to boost stem cell research
5.2.4 CHALLENGES
5.2.4.1 Lack of infrastructure for stem cell research in emerging economies
5.2.4.2 Dearth of trained and skilled professionals
5.3 RANGES/SCENARIOS
5.4 IMPACT OF COVID-19 ON STEM CELL ASSAYS MARKET
5.4.1 INTRODUCTION
5.4.2 RESEARCH & DEVELOPMENT
5.4.3 RESEARCH FUNDING
5.4.4 SUPPLY CHAIN
5.5 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESS
5.6 PRICING ANALYSIS
5.6.1 AVERAGE SELLING PRICES OF PRODUCTS OFFERED BY KEY PLAYERS
5.6.2 AVERAGE SELLING PRICE TREND
5.7 TECHNOLOGY ANALYSIS
5.7.1 MICROFABRICATION-ASSISTED TECHNOLOGIES
5.7.1.1 Microfluidics-based cell trap technology
5.7.1.2 Droplet-based microfluidic technology
5.7.1.3 Microwell-based analysis systems
5.7.2 ADVANCED TECHNOLOGIES
5.7.2.1 Bead-based multiplex assays
5.8 VALUE CHAIN ANALYSIS
5.9 SUPPLY CHAIN ANALYSIS
5.10 ECOSYSTEM ANALYSIS
5.11 PATENT ANALYSIS
5.12 KEY CONFERENCES AND EVENTS IN 2022–2023
5.13 REGULATORY ANALYSIS
5.13.1 LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS IN DIFFERENT REGIONS/COUNTRIES
5.13.2 REGULATORY ANALYSIS FOR STEM CELL RESEARCH
5.13.2.1 US
5.13.2.2 EUROPE
5.13.2.2.1 Germany
5.13.2.2.2 UK
5.13.2.2.3 France
5.13.2.2.4 Italy
5.13.2.2.5 Spain
5.13.2.2.6 Switzerland
5.13.3 REGULATORY ANALYSIS FOR STEM CELL THERAPY
5.13.3.1 US
5.13.3.2 European Union
5.14 PORTER’S FIVE FORCES ANALYSIS
5.14.1 THREAT OF NEW ENTRANTS
5.14.2 THREAT OF SUBSTITUTES
5.14.3 BARGAINING POWER OF BUYERS
5.14.4 BARGAINING POWER OF SUPPLIERS
5.14.5 DEGREE OF COMPETITION
5.15 KEY STAKEHOLDERS AND BUYING CRITERIA
5.15.1 KEY STAKEHOLDERS IN PHARMACEUTICAL COMPANIES AND THEIR INFLUENCE IN BUYING PROCESS
5.15.2 KEY BUYING CRITERIA FOR STEM CELL ASSAY PRODUCTS A MONG END USERS
6 STEM CELL ASSAYS MARKET, BY TYPE (Page No. - 84)
6.1 INTRODUCTION
6.2 VIABILITY/CYTOTOXICITY ASSAYS
6.2.1 TETRAZOLIUM REDUCTION ASSAYS
6.2.1.1 Growing use of tetrazolium reduction assays in high-throughput screening to drive segment growth
6.2.2 RESAZURIN CELL VIABILITY ASSAYS
6.2.2.1 Several advantages of resazurin cell viability assays to increase their adoption
6.2.3 CALCEIN-AM CELL VIABILITY ASSAYS
6.2.3.1 Wide applications of Calcein-AM cell viability assays across various assay techniques to drive segment growth
6.2.4 OTHER VIABILITY/CYTOTOXICITY ASSAYS
6.3 ISOLATION & PURIFICATION ASSAYS
6.3.1 GROWING USE OF ISOLATION & PURIFICATION IN STEM CELL ANALYSIS TO DRIVE SEGMENT GROWTH
6.4 CELL IDENTIFICATION ASSAYS
6.4.1 CELL IDENTIFICATION ASSAYS IDENTIFY TARGET CELLS THROUGH MARKERS, BUFFERS, AND REAGENTS
6.5 PROLIFERATION ASSAYS
6.5.1 CELL PROLIFERATION IS MEASURED ON THE BASIS OF AVERAGE DNA CONTENT AND CELLULAR METABOLISM
6.6 DIFFERENTIATION ASSAYS
6.6.1 AVAILABILITY OF VARIOUS STEM CELL DIFFERENTIAL ASSAY KITS TO BOOST SEGMENT GROWTH
6.7 FUNCTION ASSAYS
6.7.1 RISING DEMAND FOR FUNCTION ASSAYS IN DRUG DISCOVERY TO DRIVE SEGMENT GROWTH
6.8 APOPTOSIS ASSAYS
6.8.1 CASPASE ASSAYS
6.8.1.1 Advantages offered by caspase assays to drive segment growth
6.8.2 ANNEXIN V & CELL PERMEABILITY ASSAYS
6.8.2.1 Increasing use of annexin V and cell permeability assays to drive segment growth
6.8.3 DNA FRAGMENTATION ASSAYS
6.8.3.1 DNA fragmentation assays are used to study the later stages of apoptosis
6.8.4 MITOCHONDRIAL ASSAYS
6.8.4.1 Mitochondrial assays are widely used to detect mitochondrial changes associated with apoptosis
7 STEM CELL ASSAYS MARKET, BY CELL TYPE (Page No. - 113)
7.1 INTRODUCTION
7.2 ADULT STEM CELLS
7.2.1 MESENCHYMAL STEM CELLS
7.2.1.1 Increasing adoption of MSCs as potential treatment for various conditions to drive market growth
7.2.2 INDUCED PLURIPOTENT STEM CELLS
7.2.2.1 Increased adoption of iPSCs in research on cell therapies & regenerative medicine to support market growth
7.2.3 HEMATOPOIETIC STEM CELLS
7.2.3.1 Increasing focus on developing HSC-based therapies to drive market growth
7.2.4 UMBILICAL CORD STEM CELLS
7.2.4.1 Rising investments and collaborations for developing umbilical cord stem cell therapies to propel market growth
7.2.5 NEURAL STEM CELLS
7.2.5.1 Increasing research to evaluate the potency of NSCs in disease treatment will drive segment growth
7.3 HUMAN EMBRYONIC STEM CELLS
7.3.1 ESCS CAN BE STORED AND PROCESSED EASILY
8 STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE (Page No. - 129)
8.1 INTRODUCTION
8.2 INSTRUMENTS
8.2.1 FLOW CYTOMETERS
8.2.1.1 Technological advancements in flow cytometry to drive segment growth
8.2.2 CELL IMAGING & ANALYSIS SYSTEMS
8.2.2.1 High demand for cell imaging and analysis in cell behavior research studies to support segment growth
8.2.3 MICROELECTRODE ARRAYS
8.2.3.1 Increasing use of microelectrode arrays in stem cell research to drive segment growth
8.2.4 AUTOMATED CELL COUNTERS
8.2.4.1 Increasing adoption of automated cell counters to drive segment growth
8.3 KITS
8.3.1 RAPID EVOLUTION OF ASSAY PLATFORMS AND EMERGENCE OF NEW TECHNOLOGY PLATFORMS TO DRIVE SEGMENT GROWTH
8.4 SERVICES
8.4.1 RISING DEMAND FOR ASSAY DEVELOPMENT SERVICES TO DRIVE SEGMENT GROWTH
9 STEM CELL ASSAYS MARKET, BY APPLICATION (Page No. - 145)
9.1 INTRODUCTION
9.2 REGENERATIVE MEDICINE & THERAPY DEVELOPMENT
9.2.1 ORTHOPEDIC, MUSCULOSKELETAL, AND SPINE APPLICATIONS
9.2.1.1 Increasing prevalence of orthopedic disorders to drive segment growth
9.2.2 DERMATOLOGY APPLICATIONS
9.2.2.1 Increasing use of stem cells in skin disease treatment to drive segment growth
9.2.3 CARDIOVASCULAR APPLICATIONS
9.2.3.1 Growing demand for effective cardiovascular disease treatments and rising number of R&D activities to drive segment growth
9.2.4 CENTRAL NERVOUS SYSTEM APPLICATIONS
9.2.4.1 Promising stem cell therapies for degenerative neurological disorders to drive segment growth
9.2.5 ONCOLOGY APPLICATIONS
9.2.5.1 Increasing prevalence of cancer to drive segment growth
9.2.6 DIABETES APPLICATIONS
9.2.6.1 Rising incidence of diabetes to drive segment growth
9.2.7 OTHER REGENERATIVE MEDICINE & THERAPY DEVELOPMENT APPLICATIONS
9.3 DRUG DISCOVERY & DEVELOPMENT
9.3.1 RECENT PROGRESS IN THE DEVELOPMENT OF STEM CELL ASSAYS TO DRIVE SEGMENT GROWTH
9.4 CLINICAL RESEARCH
9.4.1 INCREASING NUMBER OF PROMISING CLINICAL TRIALS USING STEM CELLS TO BOOST SEGMENT GROWTH
10 STEM CELL ASSAYS MARKET, BY END USER (Page No. - 168)
10.1 INTRODUCTION
10.2 BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
10.2.1 BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES ARE LARGEST END USERS OF STEM CELL ASSAYS
10.3 ACADEMIC & RESEARCH INSTITUTES
10.3.1 NEW STEM CELL RESEARCH INSTITUTES ARE BEING ESTABLISHED FOR STEM CELL RESEARCH AND REGENERATIVE MEDICINE
11 STEM CELL ASSAYS MARKET, BY REGION (Page No. - 174)
11.1 INTRODUCTION
11.2 NORTH AMERICA
11.2.1 US
11.2.1.1 Rising funding for stem cell research to drive market growth
11.2.2 CANADA
11.2.2.1 Rising government investments & funding to drive market in Canada
11.3 EUROPE
11.3.1 GERMANY
11.3.1.1 Growing biopharma industry and focus on regenerative medicine to drive market growth
11.3.2 UK
11.3.2.1 Focus on innovation will increase demand for stem cell-based therapeutics
11.3.3 FRANCE
11.3.3.1 Government initiatives to support market growth in France
11.3.4 ITALY
11.3.4.1 Investment in R&D for development of new therapeutics to boost stem cell assays market
11.3.5 SPAIN
11.3.5.1 Increasing investments from private and public organizations to drive market growth
11.3.6 SWITZERLAND
11.3.6.1 Growing life science research to drive market growth
11.3.7 REST OF EUROPE
11.4 ASIA PACIFIC
11.4.1 CHINA
11.4.1.1 Growing focus on stem cell research to drive market growth in China
11.4.2 JAPAN
11.4.2.1 Growing collaborations for stem cell research to drive market growth in the country
11.4.3 INDIA
11.4.3.1 Government initiatives supporting stem cell research to drive market growth in India
11.4.4 REST OF ASIA PACIFIC
11.5 LATIN AMERICA
11.5.1 GROWTH OF BIOPHARMACEUTICAL INDUSTRY TO DRIVE MARKET GROWTH IN LATIN AMERICA
11.6 MIDDLE EAST & AFRICA
11.6.1 INCREASING COLLABORATIONS FOR STEM CELL RESEARCH TO DRIVE MARKET GROWTH IN MEA
12 COMPETITIVE LANDSCAPE (Page No. - 250)
12.1 INTRODUCTION
12.2 RIGHT-TO-WIN APPROACHES ADOPTED BY KEY PLAYERS
12.3 MARKET SHARE ANALYSIS
12.4 REVENUE ANALYSIS
12.5 COMPANY EVALUATION QUADRANT
12.5.1 STARS
12.5.2 EMERGING LEADERS
12.5.3 PERVASIVE PLAYERS
12.5.4 PARTICIPANTS
12.6 COMPETITIVE BENCHMARKING OF TOP 25 PLAYERS
12.6.1 OVERALL FOOTPRINT OF COMPANIES (25 COMPANIES)
12.6.2 PRODUCT & SERVICE FOOTPRINT OF COMPANIES (25 COMPANIES)
12.6.3 REGIONAL FOOTPRINT OF COMPANIES (25 COMPANIES)
12.7 COMPANY EVALUATION QUADRANT: STARTUPS/SMES
12.7.1 PROGRESSIVE COMPANIES
12.7.2 STARTING BLOCKS
12.7.3 RESPONSIVE COMPANIES
12.7.4 DYNAMIC COMPANIES
12.7.4.1 COMPETITIVE BENCHMARKING OF STARTUP/SME PLAYERS
12.8 COMPETITIVE SCENARIO AND TRENDS
12.8.1 PRODUCT LAUNCHES
12.8.2 DEALS
12.8.3 OTHER DEVELOPMENTS
13 COMPANY PROFILES (Page No. - 266)
13.1 KEY PLAYERS
13.1.1 THERMO FISHER SCIENTIFIC INC.
13.1.1.1 Business overview
13.1.1.2 Products & services offered
13.1.1.3 Recent developments
13.1.1.4 MnM view
13.1.1.4.1 Key strengths/right to win
13.1.1.4.2 Strategic choices
13.1.1.4.3 Weaknesses and competitive threats
13.1.2 MERK KGAA
13.1.2.1 Business overview
13.1.2.2 Products offered
13.1.2.3 Recent developments
13.1.2.4 MnM view
13.1.2.4.1 Key strengths/right to win
13.1.2.4.2 Strategic choices
13.1.2.4.3 Weaknesses and competitive threats
13.1.3 DANAHER
13.1.3.1 Business overview
13.1.3.2 Products offered
13.1.3.3 Recent developments
13.1.3.4 MnM view
13.1.3.4.1 Key strengths/right to win
13.1.3.4.2 Strategic choices
13.1.3.4.3 Weaknesses and competitive threats
13.1.4 BECTON, DICKINSON AND COMPANY
13.1.4.1 Business overview
13.1.4.2 Products offered
13.1.4.3 MnM view
13.1.4.3.1 Key strengths/right to win
13.1.4.3.2 Strategic choices
13.1.4.3.3 Weaknesses and competitive threats
13.1.5 BIO-RAD LABORATORIES
13.1.5.1 Business overview
13.1.5.2 Products offered
13.1.5.3 Recent developments
13.1.5.4 MnM view
13.1.5.4.1 Key strengths/right to win
13.1.5.4.2 Strategic choices
13.1.5.4.3 Weaknesses and competitive threats
13.1.6 PERKINELMER INC.
13.1.6.1 Business overview
13.1.6.2 Products offered
13.1.6.3 Recent developments
13.1.7 AGILENT TECHNOLOGIES, INC.
13.1.7.1 Business overview
13.1.7.2 Products offered
13.1.7.3 Recent developments
13.1.8 BIO-TECHNE CORPORATION
13.1.8.1 Business overview
13.1.8.2 Products & services offered
13.1.8.3 Recent development
13.1.9 FUJIFILM HOLDINGS CORPORATION
13.1.9.1 Business overview
13.1.9.2 Products & services offered
13.1.9.3 Recent developments
13.1.10 CHARLES RIVER LABORATORIES
13.1.10.1 Business overview
13.1.10.2 Services offered
13.1.10.3 Recent developments
13.1.11 LONZA GROUP
13.1.11.1 Business overview
13.1.11.2 Products offered
13.1.11.3 Recent developments
13.1.12 PROMEGA CORPORATION
13.1.12.1 Business overview
13.1.12.2 Products offered
13.1.12.3 Recent developments
13.1.13 MILTENYI BIOTEC
13.1.13.1 Business overview
13.1.13.2 Products offered
13.1.13.3 Recent developments
13.1.14 STEMCELL TECHNOLOGIES
13.1.14.1 Business overview
13.1.14.2 Products & services offered
13.1.14.3 Recent developments
13.1.15 HEMOGENIX INC.
13.1.15.1 Business overview
13.1.15.2 Products & services offered
13.1.16 CELL BIOLABS, INC.
13.1.16.1 Business overview
13.1.16.2 Products offered
13.2 OTHER COMPANIES
13.2.1 TAKARA BIO INC.
13.2.2 CREATIVE BIOARRAY
13.2.3 AAT BIOQUEST, INC.
13.2.4 ABCAM PLC
13.2.5 BPS BIOSCIENCE, INC.
13.2.6 ENZO BIOCHEM INC.
13.2.7 PROMOCELL GMBH
13.2.8 BIOTIUM
13.2.9 GENO TECHNOLOGY INC.
13.2.10 REACHBIO RESEARCH LABS
14 APPENDIX (Page No. - 324)
14.1 DISCUSSION GUIDE
14.2 KNOWLEDGE STORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
14.3 AVAILABLE CUSTOMIZATIONS
14.4 RELATED REPORTS
14.5 AUTHOR DETAILS
LIST OF TABLES (393 Tables)
TABLE 1 STEM CELL ASSAYS MARKET: IMPACT ANALYSIS
TABLE 2 GLOBAL CANCER INCIDENCE, 2020 VS. 2040
TABLE 3 AVERAGE SELLING PRICES OF PRODUCTS OFFERED BY KEY PLAYERS (TOP 5)
TABLE 4 SUPPLY CHAIN ECOSYSTEM
TABLE 5 STEM CELL ASSAYS MARKET: LIST OF CONFERENCES AND EVENTS
TABLE 6 STEM CELL ASSAYS MARKET: PORTER’S FIVE FORCES ANALYSIS
TABLE 7 STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 8 VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 9 NORTH AMERICA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 10 EUROPE: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 11 ASIA PACIFIC: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 12 VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 13 TETRAZOLIUM REDUCTION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 14 NORTH AMERICA: TETRAZOLIUM REDUCTION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 15 EUROPE: TETRAZOLIUM REDUCTION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 16 ASIA PACIFIC: TETRAZOLIUM REDUCTION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 17 RESAZURIN CELL VIABILITY ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 18 NORTH AMERICA: RESAZURIN CELL VIABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 19 EUROPE: RESAZURIN CELL VIABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 20 ASIA PACIFIC: RESAZURIN CELL VIABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 21 CALCEIN-AM CELL VIABILITY ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 22 NORTH AMERICA: CALCEIN-AM CELL VIABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 23 EUROPE: CALCEIN-AM CELL VIABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 24 ASIA PACIFIC: CALCEIN-AM CELL VIABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 25 OTHER VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 26 NORTH AMERICA: OTHER VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 27 EUROPE: OTHER VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 28 ASIA PACIFIC: OTHER VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 29 ISOLATION & PURIFICATION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 30 NORTH AMERICA: ISOLATION & PURIFICATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 31 EUROPE: ISOLATION & PURIFICATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 32 ASIA PACIFIC: ISOLATION & PURIFICATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 33 CELL IDENTIFICATION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 34 NORTH AMERICA: CELL IDENTIFICATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 35 EUROPE: CELL IDENTIFICATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 36 ASIA PACIFIC: CELL IDENTIFICATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 37 PROLIFERATION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 38 NORTH AMERICA: PROLIFERATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 39 EUROPE: PROLIFERATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 40 ASIA PACIFIC: PROLIFERATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 41 DIFFERENTIATION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 42 NORTH AMERICA: DIFFERENTIATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 43 EUROPE: DIFFERENTIATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 44 ASIA PACIFIC: DIFFERENTIATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 45 FUNCTION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 46 NORTH AMERICA: FUNCTION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 47 EUROPE: FUNCTION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 48 ASIA PACIFIC: FUNCTION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 49 APOPTOSIS ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 50 NORTH AMERICA: APOPTOSIS ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 51 EUROPE: APOPTOSIS ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 52 ASIA PACIFIC: APOPTOSIS ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 53 APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 54 CASPASE ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 55 NORTH AMERICA: CASPASE ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 56 EUROPE: CASPASE ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 57 ASIA PACIFIC: CASPASE ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 58 ANNEXIN V & CELL PERMEABILITY ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 59 NORTH AMERICA: ANNEXIN V & CELL PERMEABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 60 EUROPE: ANNEXIN V & CELL PERMEABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 61 ASIA PACIFIC: ANNEXIN V & CELL PERMEABILITY ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 62 DNA FRAGMENTATION ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 63 NORTH AMERICA: DNA FRAGMENTATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 64 EUROPE: DNA FRAGMENTATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 65 ASIA PACIFIC: DNA FRAGMENTATION ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 66 MITOCHONDRIAL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 67 NORTH AMERICA: MITOCHONDRIAL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 68 EUROPE: MITOCHONDRIAL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 69 ASIA PACIFIC: MITOCHONDRIAL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 70 STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 71 ADULT STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 72 ADULT STEM CELLS ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 73 NORTH AMERICA: ADULT STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 74 EUROPE: ADULT STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 75 ASIA PACIFIC: ADULT STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 76 MESENCHYMAL STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 77 NORTH AMERICA: MESENCHYMAL STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 78 EUROPE: MESENCHYMAL STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 79 ASIA PACIFIC: MESENCHYMAL STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 80 INDUCED PLURIPOTENT STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 81 NORTH AMERICA: INDUCED PLURIPOTENT STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 82 EUROPE: INDUCED PLURIPOTENT STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 83 ASIA PACIFIC: INDUCED PLURIPOTENT STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 84 HEMATOPOIETIC STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 85 NORTH AMERICA: HEMATOPOIETIC STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 86 EUROPE: HEMATOPOIETIC STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 87 ASIA PACIFIC: HEMATOPOIETIC STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 88 UMBILICAL CORD STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 89 NORTH AMERICA: UMBILICAL CORD STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 90 EUROPE: UMBILICAL CORD STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 91 ASIA PACIFIC: UMBILICAL CORD STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 92 NEURAL STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 93 NORTH AMERICA: NEURAL STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 94 EUROPE: NEURAL STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 95 ASIA PACIFIC: NEURAL STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 96 HUMAN EMBRYONIC STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 97 NORTH AMERICA: HUMAN EMBRYONIC STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 98 EUROPE: HUMAN EMBRYONIC STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 99 ASIA PACIFIC: HUMAN EMBRYONIC STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 100 STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 101 STEM CELL ASSAY INSTRUMENTS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 102 NORTH AMERICA: STEM CELL ASSAY INSTRUMENTS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 103 EUROPE: STEM CELL ASSAY INSTRUMENTS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 104 ASIA PACIFIC: STEM CELL ASSAY INSTRUMENTS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 105 STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 106 FLOW CYTOMETERS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 107 NORTH AMERICA: FLOW CYTOMETERS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 108 EUROPE: FLOW CYTOMETERS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 109 ASIA PACIFIC: FLOW CYTOMETERS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 110 CELL IMAGING & ANALYSIS SYSTEMS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 111 NORTH AMERICA: CELL IMAGING & ANALYSIS SYSTEMS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 112 EUROPE: CELL IMAGING & ANALYSIS SYSTEMS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 113 ASIA PACIFIC: CELL IMAGING & ANALYSIS SYSTEMS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 114 MICROELECTRODE ARRAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 115 NORTH AMERICA: MICROELECTRODE ARRAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 116 EUROPE: MICROELECTRODE ARRAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 117 ASIA PACIFIC: MICROELECTRODE ARRAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 118 AUTOMATED CELL COUNTERS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 119 NORTH AMERICA: AUTOMATED CELL COUNTERS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 120 EUROPE: AUTOMATED CELL COUNTERS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 121 ASIA PACIFIC: AUTOMATED CELL COUNTERS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 122 STEM CELL ASSAY KITS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 123 NORTH AMERICA: STEM CELL ASSAY KITS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 124 EUROPE: STEM CELL ASSAY KITS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 125 ASIA PACIFIC: STEM CELL ASSAY KITS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 126 STEM CELL ASSAY SERVICES MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 127 NORTH AMERICA: STEM CELL ASSAY SERVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 128 EUROPE: STEM CELL ASSAY SERVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 129 ASIA PACIFIC: STEM CELL ASSAY SERVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 130 STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 131 STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY REGION, 2020–2027 (USD MILLION)
TABLE 132 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 133 EUROPE: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 134 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 135 STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 136 STEM CELL ASSAYS MARKET FOR ORTHOPEDIC, MUSCULOSKELETAL, AND SPINE APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 137 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR ORTHOPEDIC, MUSCULOSKELETAL, AND SPINE APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 138 EUROPE: STEM CELL ASSAYS MARKET FOR ORTHOPEDIC, MUSCULOSKELETAL, AND SPINE APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 139 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR ORTHOPEDIC, MUSCULOSKELETAL, AND SPINE APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 140 STEM CELL ASSAYS MARKET FOR DERMATOLOGY APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 141 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR DERMATOLOGY APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 142 EUROPE: STEM CELL ASSAYS MARKET FOR DERMATOLOGY APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 143 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR DERMATOLOGY APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 144 STEM CELL ASSAYS MARKET FOR CARDIOVASCULAR APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 145 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR CARDIOVASCULAR APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 146 EUROPE: STEM CELL ASSAYS MARKET FOR CARDIOVASCULAR APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 147 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR CARDIOVASCULAR APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 148 STEM CELL ASSAYS MARKET FOR CENTRAL NERVOUS SYSTEM APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 149 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR CENTRAL NERVOUS SYSTEM APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 150 EUROPE: STEM CELL ASSAYS MARKET FOR CENTRAL NERVOUS SYSTEM APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 151 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR CENTRAL NERVOUS SYSTEM APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 152 INCREASING INCIDENCE OF CANCER, BY REGION, 2020 VS. 2030 VS. 2040 (MILLION)
TABLE 153 PROJECTED INCREASE IN THE GLOBAL NUMBER OF CANCER PATIENTS, 2015–2035
TABLE 154 STEM CELL ASSAYS MARKET FOR ONCOLOGY APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 155 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR ONCOLOGY APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 156 EUROPE: STEM CELL ASSAYS MARKET FOR ONCOLOGY APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 157 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR ONCOLOGY APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 158 TOP FIVE COUNTRIES WITH THE HIGHEST NUMBER OF DIABETICS (20–79 YEARS), 2021 VS. 2045
TABLE 159 STEM CELL ASSAYS MARKET FOR DIABETES APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 160 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR DIABETES APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 161 EUROPE: STEM CELL ASSAYS MARKET FOR DIABETES APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 162 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR DIABETES APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 163 STEM CELL ASSAYS MARKET FOR OTHER REGENERATIVE MEDICINE & THERAPY DEVELOPMENT APPLICATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 164 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR OTHER REGENERATIVE MEDICINE & THERAPY DEVELOPMENT APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 165 EUROPE: STEM CELL ASSAYS MARKET FOR OTHER REGENERATIVE MEDICINE & THERAPY DEVELOPMENT APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 166 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR OTHER REGENERATIVE MEDICINE & THERAPY DEVELOPMENT APPLICATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 167 STEM CELL ASSAYS MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY REGION, 2020–2027 (USD MILLION)
TABLE 168 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 169 EUROPE: STEM CELL ASSAYS MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 170 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 171 STEM CELL ASSAYS MARKET FOR CLINICAL RESEARCH, BY REGION, 2020–2027 (USD MILLION)
TABLE 172 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR CLINICAL RESEARCH, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 173 EUROPE: STEM CELL ASSAYS MARKET FOR CLINICAL RESEARCH, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 174 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR CLINICAL RESEARCH, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 175 STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 176 STEM CELL ASSAYS MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2020–2027 (USD MILLION)
TABLE 177 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 178 EUROPE: STEM CELL ASSAYS MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 179 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 180 STEM CELL ASSAYS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2020–2027 (USD MILLION)
TABLE 181 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 182 EUROPE: STEM CELL ASSAYS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 183 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 184 STEM CELL ASSAYS MARKET, BY REGION, 2020–2027 (USD MILLION)
TABLE 185 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 186 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 187 NORTH AMERICA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 188 NORTH AMERICA: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 189 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 190 NORTH AMERICA: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 191 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 192 NORTH AMERICA: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 193 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 194 NORTH AMERICA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 195 NORTH AMERICA: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 196 US: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 197 US: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 198 US: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 199 US: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 200 US: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 201 US: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 202 US: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 203 US: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 204 US: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 205 US: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 206 CANADA: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 207 CANADA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 208 CANADA: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 209 CANADA: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 210 CANADA: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 211 CANADA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 212 CANADA: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 213 CANADA: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 214 CANADA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 215 CANADA: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 216 EUROPE: STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 217 EUROPE: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 218 EUROPE: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 219 EUROPE: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 220 EUROPE: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 221 EUROPE: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 222 EUROPE: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 223 EUROPE: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 224 EUROPE: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 225 EUROPE: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 226 EUROPE: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 227 GERMANY: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 228 GERMANY: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 229 GERMANY: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 230 GERMANY: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 231 GERMANY: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 232 GERMANY: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 233 GERMANY: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 234 GERMANY: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 235 GERMANY: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 236 GERMANY: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 237 UK: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 238 UK: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 239 UK: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 240 UK: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 241 UK: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 242 UK: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 243 UK: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 244 UK: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 245 UK: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 246 UK: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 247 FRANCE: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 248 FRANCE: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 249 FRANCE: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 250 FRANCE: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 251 FRANCE: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 252 FRANCE: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 253 FRANCE: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 254 FRANCE: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 255 FRANCE: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 256 FRANCE: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 257 ITALY: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 258 ITALY: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 259 ITALY: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 260 ITALY: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 261 ITALY: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 262 ITALY: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 263 ITALY: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 264 ITALY: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 265 ITALY: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 266 ITALY: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 267 SPAIN: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 268 SPAIN: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 269 SPAIN: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 270 SPAIN: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 271 SPAIN: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 272 SPAIN: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 273 SPAIN: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 274 SPAIN: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 275 SPAIN: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 276 SPAIN: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 277 SWITZERLAND: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 278 SWITZERLAND: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 279 SWITZERLAND: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 280 SWITZERLAND: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 281 SWITZERLAND: ADULT STEM CELLS MARKET, BY TYPE, 020–2027 (USD MILLION)
TABLE 282 SWITZERLAND: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 283 SWITZERLAND: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 284 SWITZERLAND: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 285 SWITZERLAND: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 286 SWITZERLAND: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 287 REST OF EUROPE: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 288 REST OF EUROPE: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 289 REST OF EUROPE: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 290 REST OF EUROPE: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 291 REST OF EUROPE: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 292 REST OF EUROPE: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 293 REST OF EUROPE: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 294 REST OF EUROPE: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 295 REST OF EUROPE: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 296 REST OF EUROPE: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 297 ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 298 ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 299 ASIA PACIFIC: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 300 ASIA PACIFIC: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 301 ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 302 ASIA PACIFIC: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 303 ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 304 ASIA PACIFIC: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 305 ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 306 ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 307 ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 308 CHINA: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 309 CHINA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 310 CHINA: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 311 CHINA: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 312 CHINA: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 313 CHINA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 314 CHINA: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 315 CHINA: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 316 CHINA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 317 CHINA: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 318 JAPAN: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 319 JAPAN: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 320 JAPAN: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 321 JAPAN: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 322 JAPAN: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 323 JAPAN: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 324 JAPAN: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 325 JAPAN: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 326 JAPAN: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 327 JAPAN: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 328 INDIA: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 329 INDIA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 330 INDIA: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 331 INDIA: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 332 INDIA: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 333 INDIA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 334 INDIA: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 335 INDIA: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 336 INDIA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 337 INDIA: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 338 REST OF ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 339 REST OF ASIA PACIFIC: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 340 REST OF ASIA PACIFIC: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 341 REST OF ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 342 REST OF ASIA PACIFIC: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 343 REST OF ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 344 REST OF ASIA PACIFIC: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 345 REST OF ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 346 REST OF ASIA PACIFIC: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 347 REST OF ASIA PACIFIC: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 348 MEXICO: CANCER CASES, BY CANCER TYPE, 2020
TABLE 349 LATIN AMERICA: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 350 LATIN AMERICA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 351 LATIN AMERICA: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 352 LATIN AMERICA: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 353 LATIN AMERICA: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 354 LATIN AMERICA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 355 LATIN AMERICA: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 356 LATIN AMERICA: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 357 LATIN AMERICA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 358 LATIN AMERICA: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 359 MIDDLE EAST & AFRICA: STEM CELL ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 360 MIDDLE EAST & AFRICA: VIABILITY/CYTOTOXICITY ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 361 MIDDLE EAST & AFRICA: APOPTOSIS ASSAYS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 362 MIDDLE EAST & AFRICA: STEM CELL ASSAYS MARKET, BY CELL TYPE, 2020–2027 (USD MILLION)
TABLE 363 MIDDLE EAST & AFRICA: ADULT STEM CELLS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 364 MIDDLE EAST & AFRICA: STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2020–2027 (USD MILLION)
TABLE 365 MIDDLE EAST & AFRICA: STEM CELL ASSAY INSTRUMENTS MARKET, BY TYPE, 2020–2027 (USD MILLION)
TABLE 366 MIDDLE EAST & AFRICA: STEM CELL ASSAYS MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
TABLE 367 MIDDLE EAST & AFRICA: STEM CELL ASSAYS MARKET FOR REGENERATIVE MEDICINE & THERAPY DEVELOPMENT, BY TYPE, 2020–2027 (USD MILLION)
TABLE 368 MIDDLE EAST & AFRICA: STEM CELL ASSAYS MARKET, BY END USER, 2020–2027 (USD MILLION)
TABLE 369 STEM CELL ASSAYS MARKET: DEGREE OF COMPETITION
TABLE 370 PRODUCT & REGIONAL FOOTPRINT ANALYSIS OF KEY PLAYERS IN STEM CELL ASSAYS MARKET
TABLE 371 PRODUCT & SERVICE FOOTPRINT ANALYSIS OF KEY PLAYERS IN STEM CELL ASSAYS MARKET
TABLE 372 REGIONAL FOOTPRINT ANALYSIS OF KEY PLAYERS IN STEM CELL ASSAYS MARKET
TABLE 373 STEM CELL ASSAYS MARKET: DETAILED LIST OF KEY STARTUPS/SMES
TABLE 374 STEM CELL ASSAYS MARKET: COMPETITIVE BENCHMARKING OF KEY PLAYERS (STARTUPS/SMES)
TABLE 375 STEM CELL ASSAYS MARKET: PRODUCT LAUNCHES, JANUARY 2018-MAY 2022
TABLE 376 STEM CELL ASSAYS MARKET: DEALS, JANUARY 2018-MAY 2022
TABLE 377 STEM CELL ASSAYS MARKET: OTHER DEVELOPMENTS, JANUARY 2018-MAY 2022
TABLE 378 THERMO FISHER SCIENTIFIC INC: BUSINESS OVERVIEW
TABLE 379 MERCK KGAA: BUSINESS OVERVIEW
TABLE 380 DANAHER: BUSINESS OVERVIEW
TABLE 381 BECTON, DICKINSON AND COMPANY: BUSINESS OVERVIEW
TABLE 382 BIO-RAD LABORATORIES: BUSINESS OVERVIEW
TABLE 383 PERKINELMER INC.: BUSINESS OVERVIEW
TABLE 384 AGILENT TECHNOLOGIES, INC.: BUSINESS OVERVIEW
TABLE 385 BIO-TECHNE CORPORATION: BUSINESS OVERVIEW
TABLE 386 FUJIFILM HOLDINGS CORPORATION: BUSINESS OVERVIEW
TABLE 387 CHARLES RIVER LABORATORIES: BUSINESS OVERVIEW
TABLE 388 LONZA GROUP: BUSINESS OVERVIEW
TABLE 389 PROMEGA CORPORATION: BUSINESS OVERVIEW
TABLE 390 MILTENYI BIOTEC: BUSINESS OVERVIEW
TABLE 391 STEMCELL TECHNOLOGIES: BUSINESS OVERVIEW
TABLE 392 HEMOGENIX INC.: BUSINESS OVERVIEW
TABLE 393 CELL BIOLABS, INC.: BUSINESS OVERVIEW
LIST OF FIGURES (47 Figures)
FIGURE 1 RESEARCH DESIGN
FIGURE 2 BREAKDOWN OF PRIMARIES, BY RESPONDENT, DESIGNATION, AND REGION
FIGURE 3 STEM CELL ASSAYS MARKET SIZE ESTIMATION (SUPPLY-SIDE ANALYSIS), 2021
FIGURE 4 MARKET SIZE ESTIMATION: APPROACH 1 (COMPANY REVENUE ANALYSIS-BASED ESTIMATION), 2021
FIGURE 5 MARKET SIZE VALIDATION FROM PRIMARY SOURCES
FIGURE 6 STEM CELL ASSAYS MARKET (SUPPLY SIDE): CAGR PROJECTIONS
FIGURE 7 STEM CELL ASSAYS MARKET (DEMAND SIDE): GROWTH ANALYSIS OF DEMAND-SIDE FACTORS
FIGURE 8 DATA TRIANGULATION METHODOLOGY
FIGURE 9 STEM CELL ASSAYS MARKET, BY TYPE, 2022 VS. 2027 (USD MILLION)
FIGURE 10 STEM CELL ASSAYS MARKET, BY CELL TYPE, 2022 VS. 2027 (USD MILLION)
FIGURE 11 STEM CELL ASSAYS MARKET, BY PRODUCT & SERVICE, 2022 VS. 2027 (USD MILLION)
FIGURE 12 STEM CELL ASSAYS MARKET, BY APPLICATION, 2022 VS. 2027 (USD MILLION)
FIGURE 13 STEM CELL ASSAYS MARKET, BY END USER, 2022 VS. 2027 (USD MILLION)
FIGURE 14 GEOGRAPHICAL SNAPSHOT OF STEM CELL ASSAYS MARKET
FIGURE 15 RISING AWARENESS ABOUT THERAPEUTIC POTENCY OF STEM CELLS TO DRIVE MARKET GROWTH
FIGURE 16 INSTRUMENTS SEGMENT ACCOUNTED FOR LARGEST MARKET SHARE IN 2021
FIGURE 17 VIABILITY/CYTOTOXICITY ASSAYS SEGMENT WILL CONTINUE TO DOMINATE MARKET IN 2027
FIGURE 18 REGENERATIVE MEDICINE & THERAPY DEVELOPMENT SEGMENT DOMINATED MARKET IN 2021
FIGURE 19 ASIA PACIFIC COUNTRIES TO REGISTER HIGHEST GROWTH RATE FROM 2022 TO 2027
FIGURE 20 STEM CELL ASSAYS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
FIGURE 21 SPECTRUM OF SCENARIOS BASED ON IMPACT OF UNCERTAINTIES ON GROWTH OF STEM CELL ASSAYS MARKET
FIGURE 22 STEM CELL ASSAYS MARKET SCENARIO WITH AND WITHOUT COVID-19 IMPACT, 2019-2021
FIGURE 23 REVENUE SHIFT AND NEW REVENUE POCKETS FOR STEM CELL ASSAY PRODUCT PROVIDERS
FIGURE 24 VALUE CHAIN ANALYSIS OF STEM CELL ASSAYS MARKET
FIGURE 25 STEM CELL ASSAYS MARKET: SUPPLY CHAIN ANALYSIS
FIGURE 26 ECOSYSTEM ANALYSIS: STEM CELL ASSAYS MARKET
FIGURE 27 PATENT APPLICATIONS RELATED TO STEM CELL ASSAYS MARKET, JANUARY 2012–MARCH 2022
FIGURE 28 INFLUENCE OF STAKEHOLDERS IN PHARMACEUTICAL COMPANIES IN BUYING PROCESS OF STEM CELL ASSAY PRODUCTS
FIGURE 29 KEY BUYING CRITERIA FOR END USERS
FIGURE 30 NORTH AMERICA: STEM CELL ASSAYS MARKET SNAPSHOT
FIGURE 31 APAC: STEM CELL ASSAYS MARKET SNAPSHOT
FIGURE 32 STEM CELL ASSAYS MARKET: STRATEGIES ADOPTED
FIGURE 33 STEM CELL ASSAYS MARKET SHARE ANALYSIS, BY KEY PLAYER (2021)
FIGURE 34 REVENUE ANALYSIS FOR KEY COMPANIES (2019-2021)
FIGURE 35 STEM CELL ASSAYS MARKET: COMPANY EVALUATION MATRIX, 2021
FIGURE 36 STEM CELL ASSAYS MARKET: COMPANY EVALUATION MATRIX FOR STARTUPS/SMES, 2021
FIGURE 37 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2021)
FIGURE 38 MERCK KGAA: COMPANY SNAPSHOT (2021)
FIGURE 39 DANAHER: COMPANY SNAPSHOT (2021)
FIGURE 40 BECTON, DICKINSON AND COMPANY: COMPANY SNAPSHOT (2021)
FIGURE 41 BIO-RAD LABORATORIES: COMPANY SNAPSHOT (2021)
FIGURE 42 PERKINELMER INC.: COMPANY SNAPSHOT (2021)
FIGURE 43 AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT (2021)
FIGURE 44 BIO-TECHNE CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 45 FUJIFILM HOLDINGS CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 46 CHARLES RIVER LABORATORIES: COMPANY SNAPSHOT (2021)
FIGURE 47 LONZA GROUP: COMPANY SNAPSHOT (2021)
This study involved four major activities in estimating the current size of the stem cell assays market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate the market size of segments and subsegments.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the stem cell assays market. The secondary sources used for this study include International Society for Stem Cell Research (ISSCR), International Stem Cell Forum (ISCF), International Society for Cellular Therapy (ISCT), Council for International Organizations of Medical Sciences (CIOMS), International Consortium of Stem Cell Networks (ICSCN), United States Food and Drug Administration (US FDA), National Institutes of Health (US), National Center for Biotechnology Information (NCBI), EuroStemCell, European Medicines Agency (EMA), Clinical Trials Registry, American Society for Cell Biology (ASCB), Center for Commercialization of Regenerative Medicine (CCRM), and International Agency for Research on Cancer (IARC), Annual Reports, SEC Filings, Investor Presentations, Expert Interviews, and MarketsandMarkets Analysis. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
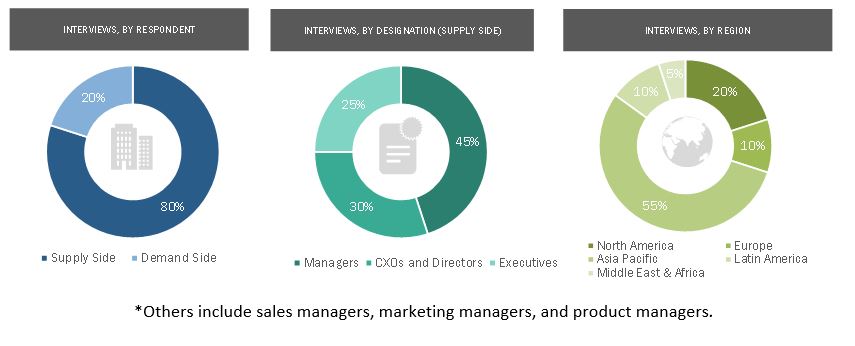
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information. The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the stem cell assays market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and market have been identified through extensive secondary research
- The revenues generated from the stem cell assays and related products business of leading players have been determined through primary and secondary research
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
Data Triangulation
After arriving at the overall market size from the market size estimation process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Report Objectives
- To define, describe, and forecast the global stem cell assays market based on the product, functionslity, formulation and region
- To provide detailed information regarding the major factors influencing the growth of the market (such as drivers, restraints, challenges, and opportunities)
- To strategically analyze micromarkets with respect to individual growth trends, future prospects, and contributions to the overall stem cell assays market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five main regions, namely, North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa
- To strategically profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments such as acquisitions, product launches, expansions, agreements, partnerships, and R&D activities in the stem cell assays market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Company Information
- An additional five company profiles



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Stem Cell Assays Market