Medical Electrodes Market by Product [Diagnostic Electrodes (ECG, EEG, EMG), Therapeutic Electrodes (Defibrillator, Pacemaker)], Technology (Wet, Dry, Needle), Application (Neurophysiology, IOM), Usage (Disposable, Reusable) & Region - Global Forecast to 2026
Market Growth Outlook Summary
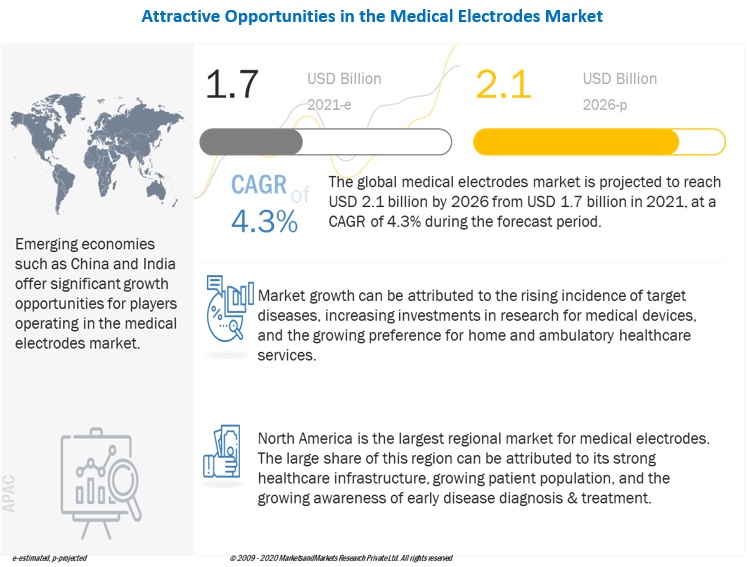
The global medical electrodes market growth forecasted to transform from $1.7 billion in 2021 to $2.1 billion by 2026, driven by a CAGR of 4.3%. Growth in this market is primarily driven by the increase in the incidence rate of neurological and cardiovascular disorders and the increasing preference for home and ambulatory care services.
Medical Electrodes Market Trends
To know about the assumptions considered for the study, download the pdf brochure
Medical Electrodes Market Dynamics
Driver: Rising incidence of neurological & cardiovascular disorders
The incidence of neurological, cardiovascular, and sleep disorders has significantly increased over the years. This increase has, in turn, accelerated the number of diagnostic and treatment procedures conducted. Thus, the increasing demand for these procedures is likely to support market growth. For instance, according to the World Health Organization (WHO), cardiovascular disease (CVD) is a leading cause of death and disability worldwide. Globally, 17.9 million deaths due to CVD were reported in 2016; this figure is projected to increase to 23.6 million deaths by 2030.
Opportunity: Growth opportunities in emerging economies
Emerging economies such as BRIC (Brazil, Russia, India, and China) and countries in Latin America and Southeast Asia are expected to provide significant growth opportunities to players operating in the global market. This can be attributed in large part to the low competition in these markets, the large population, the rising number of cardiovascular and neurological diseases, and an increase in disposable income.
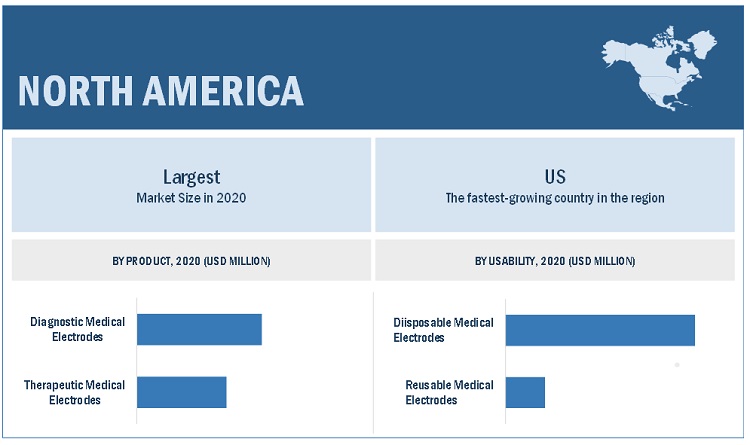
The diagnostic medical electrodes segment accounted for the largest share of the medical electrodes industry, by product
Based on product, the medical electrodes market is segmented into diagnostic medical electrodes and therapeutic medical electrodes. The diagnostic medical electrodes segment accounted for the largest share of the global market. The large share of this segment is primarily due to the rising emphasis on preventive medicine, coupled with rising the number of patients with chronic diseases.
The wet electrode segment dominated the medical electrodes industry, by technology.
Based on technology, the medical electrodes market is segmented into dry electrodes, wet electrodes, and needle electrodes. The wet electrodes segment accounted for the largest share of the global market. The large share of this segment can be attributed to the rising preference for the high signal quality that is offered efficiently by wet electrodes.
The disposable electrodes segment is expected to witness the highest CAGR in the medical electrodes industry by usability during the forecast period.
The medical electrodes market is segmented into disposable electrodes and reusable electrodes, based on usability. In 2020, the disposable electrodes segment accounted for the largest share and is also expected to register a higher growth rate during the forecast period. The large share of the disposable electrodes segment is due to factors such as the rising adoption of disposable electrodes due to the low risk of cross-contamination and cost-effectiveness associated with disposable electrodes.
The cardiology segment accounted for the largest share by application in the medical electrode industry
Based on application, the medical electrodes market is segmented into cardiology, neurophysiology, sleep disorders, intraoperative monitoring (IOM), surgical applications, and other applications. Factors such as the increasing prevalence of cardiovascular diseases (CVD), rising cardiovascular health awareness, and technological innovations in diagnostics tools have accelerated the growth of the segment.
North America was the largest region for medical electrodes industry.
The medical electrodes market is segmented into North America, Europe, the Asia Pacific, and the Rest of the World. In 2020, North America was the largest regional segment of the overall market, followed by Europe. The large share of the North American market is due to factors such as the, rising geriatric population, the growing incidence of chronic diseases, and the increasing focus on innovative technologies by medical electrode manufacturers.
To know about the assumptions considered for the study, download the pdf brochure
The prominent players operating in the medical electrodes market include Medtronic (Ireland), Ambu A/S (Denmark), 3M (US), Natus Medical Incorporated (US), Koninklijke Philips N.V. (Netherlands), B. Braun Melsungen AG (Germany), ZOLL Medical Corporation (US), Cardinal Health (US), CONMED Corporation (US), Nihon Kohden Corporation (Japan), Rhythmlink International, LLC (US), Cognionics, Inc. (US), GE Healthcare (US), Compumedics Limited (Australia), and Nissha Medical Technologies (Japan).
Scope of the Medical Electrodes Industry
|
Report Metric |
Details |
|
Market Revenue Size in 2021 |
$1.7 billion |
|
Projected Revenue Size by 2026 |
$2.1 billion |
|
Industry Growth Rate |
Poised to Grow at a CAGR of 3.6% |
|
Market Driver |
Rising incidence of neurological & cardiovascular disorders |
|
Market Opportunity |
Growth opportunities in emerging economies |
This report categorizes the global medical electrodes market to forecast revenue and analyze trends in each of the following submarkets:
By Product
-
Diagnostic Medical Electrodes
- Electrocardiography (ECG) Electrodes
- Electroencephalography (EEG) Electrodes
- Electromyography (EMG) Electrodes
- Other Diagnostic Electrodes [electroretinography (ERG) electrodes and electrooculography (EOG) electrodes]
-
Therapeutic Medical Electrodes
- Defibrillator Electrodes
- Pacemaker Electrodes
- TENS Electrodes
- Electrosurgical Electrodes
- Neuromuscular Stimulation Electrodes (NMES)
- Other Therapeutic Electrodes (wound healing electrodes, blood gas electrodes, and iontophoresis electrodes)
By Technology
- Wet Electrodes
- Dry Electrodes
- Needle Electrodes
By Usability
- Disposable Medical Electrodes
- Reusable Medical Electrodes
By Application
- Cardiology
- Neurophysiology
- Sleep Disorders
- Intraoperative Monitoring (IOM)
- Surgical Applications
- Other Applications (vision disorders and wound healing)
By Region
-
North America
- US
- Canada
-
Europe
- Germany
- France
- UK
- RoE
-
Asia Pacific
- Japan
- China
- India
- Rest of Asia Pacific
- Rest of the World
Recent Developments of Medical Electrodes Industry:
- In 2021, Sticky Pad Surface Electrodes manufacturered by Rhythmlink International, LLC (US) received FDA clearance for MR Conditional for 1.5 and 3 Tesla MRI environments and now can be used safely and effectively in imaging environments.
- In 2020, Natus Medical, Inc. (US) entered into a partnership with Holberg EEG AS (Norway). This partnership aims to improve and automate the diagnosis of epilepsy by developing and distributing an AutoSCORE algorithm worldwide.
- In 2019, Medtronic (Ireland) entered into a distribution agreement with Alpha Omega (Israel). This agreement aimed to market Medtronic’s surgical navigation products designed for procedures in the brain.
Frequently Asked Questions (FAQs):
What is the projected market value of the global Medical Electrodes Market?
The global market of Medical Electrodes Market is projected to reach USD 2.1 Billion.
What is the estimated growth rate (CAGR) of the global Medical Electrodes Market for the next five years?
The global Medical Electrodes Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 4.3% from 2021 to 2026.
What are the major revenue pockets in the Medical Electrodes Market currently?
North America was the largest regional segment of the overall market, followed by Europe. The large share of the North American market is due to factors such as the, rising geriatric population, the growing incidence of chronic diseases, and the increasing focus on innovative technologies by medical electrode manufacturers.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst

TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 26)
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.2.1 INCLUSION & EXCLUSIONS
1.3 MARKET SCOPE
FIGURE 1 MEDICAL ELECTRODES MARKET
1.3.1 YEARS CONSIDERED FOR THE STUDY
1.4 CURRENCY
1.5 LIMITATIONS
1.6 STAKEHOLDERS
1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY (Page No. - 30)
2.1 RESEARCH DATA
FIGURE 2 RESEARCH DESIGN
2.1.1 SECONDARY DATA
2.1.1.1 Secondary sources
2.1.2 PRIMARY DATA
FIGURE 3 PRIMARY SOURCES
2.1.2.1 Key data from primary sources
2.1.2.2 Key industry insights
FIGURE 4 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
2.2 MARKET SIZE ESTIMATION
FIGURE 5 MARKET SIZE ESTIMATION: BOTTOM-UP APPROACH
2.2.1 GROWTH FORECAST
FIGURE 6 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
FIGURE 7 TOP-DOWN APPROACH
2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
FIGURE 8 DATA TRIANGULATION METHODOLOGY
2.4 ASSUMPTIONS FOR THE STUDY
2.5 COVID-19 ECONOMIC ASSESSMENT
2.6 ASSESSMENT OF THE IMPACT OF COVID-19 ON THE ECONOMIC SCENARIO
FIGURE 9 CRITERIA IMPACTING THE GLOBAL ECONOMY
FIGURE 10 RECOVERY SCENARIO OF THE GLOBAL ECONOMY
3 EXECUTIVE SUMMARY (Page No. - 42)
3.1 INTRODUCTION
FIGURE 11 MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2021 VS. 2026
FIGURE 12 GLOBAL MARKET, BY TECHNOLOGY, 2021 VS. 2026
FIGURE 13 GLOBAL MARKET, BY USABILITY, 2021–2026
FIGURE 14 GLOBAL MARKET, BY APPLICATION, 2021 VS. 2026
FIGURE 15 GEOGRAPHIC SNAPSHOT OF THE GLOBAL MEDICAL ELECTRODES INDUSTRY
4 PREMIUM INSIGHTS (Page No. - 46)
4.1 GLOBAL MARKET OVERVIEW
FIGURE 16 RISING INCIDENCE OF NEUROLOGICAL DISORDERS & CVD TO DRIVE MARKET GROWTH DURING THE FORECAST PERIOD
4.2 ASIA PACIFIC: MARKET, BY DIAGNOSTIC MEDICAL ELECTRODES & COUNTRY (2021)
FIGURE 17 ELECTROCARDIOGRAPHY (ECG) ELECTRODES TO ACCOUNT FOR THE LARGEST SHARE, BY DIAGNOSTIC ELECTRODES, IN 2021
4.3 GLOBAL MARKET, BY PRODUCT, 2021-2026
FIGURE 18 DIAGNOSTIC MEDICAL ELECTRODES ARE EXPECTED TO DOMINATE THE MARKET DURING THE FORECAST PERIOD
4.4 GLOBAL MARKET: GEOGRAPHIC SNAPSHOT
FIGURE 19 CHINA TO REGISTER THE HIGHEST GROWTH RATE DURING THE FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 49)
5.1 INTRODUCTION
5.2 MEDICAL ELECTRODES INDUSTRY DYNAMICS
FIGURE 20 GLOBAL MARKET: DRIVERS AND OPPORTUNITIES
5.2.1 DRIVERS
5.2.1.1 The rising incidence of neurological & cardiovascular disorders
5.2.1.2 Increasing investments in research for medical devices
5.2.1.3 Growing preference for home & ambulatory care services
5.2.1.4 Rising demand for minimally invasive surgeries
5.2.2 OPPORTUNITIES
5.2.2.1 Growth opportunities in emerging economies
TABLE 1 ASIA: HEALTHCARE EXPENDITURE (% OF GDP), BY COUNTRY
5.3 REGULATORY LANDSCAPE
5.3.1 FDA
TABLE 2 US: REGULATORY APPROVAL PROCESS, BY DEVICE TYPE
5.3.2 EUROPEAN REGULATIONS
5.3.3 OTHER COUNTRIES
TABLE 3 MEDICAL DEVICE REGULATIONS, BY COUNTRY
5.4 COVID-19 IMPACT ON THE GLOBAL MARKET
5.5 TECHNOLOGY ANALYSIS
5.6 PRICING ANALYSIS
TABLE 4 US: PRICE OF MEDICAL ELECTRODES, BY PRODUCT
5.7 TRADE ANALYSIS
TABLE 5 IMPORT DATA FOR ELECTROCARDIOGRAPHS, BY COUNTRY, 2016-2020
TABLE 6 EXPORT DATA FOR ELECTROCARDIOGRAPHS, BY COUNTRY, 2016-2020
5.8 PATENT ANALYSIS
5.9 VALUE CHAIN ANALYSIS
FIGURE 21 VALUE CHAIN ANALYSIS: MAJOR VALUE IS ADDED DURING THE MANUFACTURING AND ASSEMBLY PHASE
5.10 SUPPLY CHAIN ANALYSIS
FIGURE 22 DISTRIBUTION: A STRATEGY PREFERRED BY PROMINENT COMPANIES
5.11 MEDICAL ELECTRODES ECOSYSTEM
FIGURE 23 MEDICAL ELECTRODES ECOSYSTEM ANALYSIS
5.11.1 ROLE IN ECOSYSTEM
5.12 YCC SHIFT
5.13 PORTER’S FIVE FORCES ANALYSIS
TABLE 7 HIGH CONSOLIDATION IN THE MARKET TO RESTRICT THE ENTRY OF NEW PLAYERS
5.13.1 DEGREE OF COMPETITION
5.13.2 BARGAINING POWER OF SUPPLIERS
5.13.3 BARGAINING POWER OF BUYERS
5.13.4 THREAT OF SUBSTITUTES
5.13.5 THREAT OF NEW ENTRANTS
6 MEDICAL ELECTRODES MARKET, BY PRODUCT (Page No. - 65)
6.1 INTRODUCTION
TABLE 8 GLOBAL MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
6.2 DIAGNOSTIC MEDICAL ELECTRODES
TABLE 9 MARKET for DIAGNOSTIC MEDICAL ELECTRODES, BY TYPE, 2019–2026
TABLE 10 MARKET for DIAGNOSTIC MEDICAL ELECTRODES , BY REGION, 2019–2026
6.2.1 ELECTROCARDIOGRAPHY (ECG) ELECTRODES
6.2.1.1 The rising prevalence of cardiovascular diseases to drive market growth for ECG electrodes
TABLE 11 ECG ELECTRODES MARKET, BY REGION, 2019–2026
6.2.2 ELECTROENCEPHALOGRAPHY (EEG) ELECTRODES
6.2.2.1 EEG electrodes help in monitoring seizure disorders and other brain conditions
TABLE 12 EEG ELECTRODES MARKET, BY REGION, 2019–2026
6.2.3 ELECTROMYOGRAPHY (EMG) ELECTRODES
6.2.3.1 Increasing incidences of neuromuscular disorders to drive the market growth
TABLE 13 EMG ELECTRODES MARKET, BY REGION, 2019–2026
6.2.4 OTHER DIAGNOSTIC ELECTRODES
TABLE 14 OTHER DIAGNOSTIC ELECTRODES MARKET, BY REGION, 2019–2026
6.3 THERAPEUTIC MEDICAL ELECTRODES
TABLE 15 THERAPEUTIC MARKET, BY TYPE, 2019–2026
TABLE 16 THERAPEUTIC ELECTRODES MARKET, BY REGION, 2019–2026
6.3.1 DEFIBRILLATOR ELECTRODES
6.3.1.1 Rising prevalence of CVD to drive the growth of this segment
TABLE 17 DEFIBRILLATOR ELECTRODES MARKET, BY REGION, 2019–2026
6.3.2 TRANSCUTANEOUS ELECTRICAL NERVE STIMULATION (TENS) ELECTRODES
6.3.2.1 Low cost and ease of use to support the market growth for this segment
TABLE 18 TENS ELECTRODES MARKET, BY REGION, 2019–2026
6.3.3 ELECTROSURGICAL ELECTRODES
6.3.3.1 Increasing demand for minimally invasive surgical procedures to drive the market growth
TABLE 19 ELECTROSURGICAL ELECTRODES MARKET, BY REGION, 2019–2026
6.3.4 PACEMAKER ELECTRODES
6.3.4.1 Rising geriatric population to drive market growth for pacemaker electrodes
TABLE 20 PACEMAKER ELECTRODES MARKET, BY REGION, 2019–2026
6.3.5 NEUROMUSCULAR ELECTRICAL STIMULATION (NMES) ELECTRODES
6.3.5.1 NMES electrodes are largely used in the field of neurorehabilitation
TABLE 21 NMES ELECTRODES MARKET, BY REGION, 2019–2026
6.3.6 OTHER THERAPEUTIC ELECTRODES
TABLE 22 OTHER THERAPEUTIC ELECTRODES MARKET, BY REGION, 2019–2026
7 MEDICAL ELECTRODES MARKET, BY TECHNOLOGY (Page No. - 78)
7.1 INTRODUCTION
TABLE 23 GLOBAL MEDICAL ELECTRODES INDUSTRY, BY TECHNOLOGY, 2019–2026
7.2 WET ELECTRODES
7.2.1 WET ELECTRODES OFFER HIGH-QUALITY SIGNAL RECORDING
TABLE 24 WET ELECTRODES MARKET, BY REGION, 2019–2026
7.3 DRY ELECTRODES
7.3.1 TECHNOLOGICAL ADVANCEMENTS IN DRY ELECTRODES PROVIDE BETTER PATIENT COMFORT
TABLE 25 SPIKE SIZE OF ELECTRODES WITH ADVANTAGES AND DISADVANTAGES
TABLE 26 COMMERCIAL EEG SYSTEMS BASED ON DRY ELECTRODES
TABLE 27 DRY ELECTRODES MARKET, BY REGION, 2019–2026
7.4 NEEDLE ELECTRODES
7.4.1 NEEDLE ELECTRODES ARE LESS SUSCEPTIBLE TO MOVEMENT ERRORS
TABLE 28 NEEDLE ELECTRODES MARKET, BY REGION, 2019–2026
8 MEDICAL ELECTRODES MARKET, BY USABILITY (Page No. - 84)
8.1 INTRODUCTION
TABLE 29 GLOBAL MEDICAL ELECTRODES INDUSTRY, BY USABILITY, 2019-2026
8.2 DISPOSABLE MEDICAL ELECTRODES
8.2.1 GROWING PREVALENCE OF HAIS TO DRIVE THE MARKET GROWTH OF THIS SEGMENT
TABLE 30 DISPOSABLE MARKET, BY REGION, 2019-2026
8.3 REUSABLE MEDICAL ELECTRODES
8.3.1 THE HIGH MAINTENANCE COST OF REUSABLE ELECTRODES LIMITS ADOPTION
TABLE 31 REUSABLE MARKET, BY REGION, 2019-2026
9 MEDICAL ELECTRODES MARKET, BY APPLICATION (Page No. - 88)
9.1 INTRODUCTION
TABLE 32 GLOBAL MEDICAL ELECTRODES INDUSTRY, BY APPLICATION, 2019-2026
9.2 CARDIOLOGY
9.2.1 INCREASING INCIDENCE OF CARDIOVASCULAR DISEASES TO DRIVE THE MARKET GROWTH
TABLE 33 GLOBAL MARKET FOR CARDIOLOGY, BY REGION, 2019-2026
9.3 NEUROPHYSIOLOGY
9.3.1 THE GROWING PREVALENCE OF BRAIN DISORDERS TO SUPPORT MARKET GROWTH FOR THIS SEGMENT
TABLE 34 GLOBAL MARKET FOR NEUROPHYSIOLOGY, BY REGION, 2019-2026
9.4 SLEEP DISORDERS
9.4.1 GROWING INCIDENCE OF SLEEP DISORDERS TO DRIVE THE GROWTH OF THIS SEGMENT
TABLE 35 GLOBAL MARKET FOR SLEEP DISORDERS, BY REGION, 2019-2026
9.5 INTRAOPERATIVE MONITORING (IOM)
9.5.1 IOM HELPS REDUCE THE RISK OF NEUROLOGICAL DEFICITS
TABLE 36 GLOBAL MARKET FOR INTRAOPERATIVE MONITORING, BY REGION, 2019-2026
9.6 SURGICAL APPLICATIONS
9.6.1 INCREASING NUMBER OF MINIMALLY INVASIVE SURGERIES TO DRIVE THE MARKET GROWTH FOR SURGICAL APPLICATIONS
TABLE 37 GLOBAL MARKET FOR SURGICAL APPLICATIONS, BY REGION, 2019-2026
9.7 OTHER APPLICATIONS
TABLE 38 GLOBAL MARKET FOR OTHER APPLICATIONS, BY REGION, 2019-2026
10 MEDICAL ELECTRODES MARKET, BY REGION (Page No. - 95)
10.1 INTRODUCTION
TABLE 39 GLOBAL MEDICAL ELECTRODES INDUSTRY, BY REGION, 2019–2026
10.2 NORTH AMERICA
FIGURE 24 NORTH AMERICA: MEDICAL ELECTRODES INDUSTRY SNAPSHOT
TABLE 40 NORTH AMERICA: MARKET, BY COUNTRY, 2019–2026
TABLE 41 NORTH AMERICA: MARKET, BY PRODUCT, 2019–2026
TABLE 42 NORTH AMERICA: DIAGNOSTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 43 NORTH AMERICA: THERAPEUTIC ELECTRODES MARKET, BY TYPE, 2019–2026
TABLE 44 NORTH AMERICA: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 45 NORTH AMERICA: MARKET, BY USABILITY, 2019–2026
TABLE 46 NORTH AMERICA: MARKET, BY APPLICATION, 2019–2026
10.2.1 US
10.2.1.1 The US accounts for the largest share of the North American market during the forecast period
TABLE 47 US: KEY MACROINDICATORS
TABLE 48 US: MARKET, BY PRODUCT, 2019–2026
TABLE 49 US: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 50 US: THERAPEUTIC MEDICAL ELECTRODES MARKET, BY PRODUCT, 2019–2026
TABLE 51 US: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 52 US: MARKET, BY USABILITY, 2019–2026
TABLE 53 US: MARKET, BY APPLICATION, 2019–2026
10.2.2 CANADA
10.2.2.1 Increasing research in the field of cardiology and neurology to drive the market
TABLE 54 RESEARCH GRANTS FOR PATIENT MONITORING DEVICES IN CANADA
TABLE 55 CANADA: KEY MACROINDICATORS
TABLE 56 CANADA: MARKET, BY PRODUCT, 2019–2026
TABLE 57 CANADA: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 58 CANADA: THERAPEUTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 59 CANADA: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 60 CANADA: MARKET, BY USABILITY, 2019–2026
TABLE 61 CANADA: MARKET, BY APPLICATION,2019–2026
10.3 EUROPE
TABLE 62 EUROPE: MARKET, BY COUNTRY, 2019–2026
TABLE 63 EUROPE: MARKET, BY PRODUCT, 2019–2026
TABLE 64 EUROPE: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 65 EUROPE: THERAPEUTIC MEDICAL ELECTRODES MARKET, BY PRODUCT, 2019–2026
TABLE 66 EUROPE: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 67 EUROPE: MARKET, BY USABILITY, 2019–2026
TABLE 68 EUROPE: MARKET, BY APPLICATION, 2019–2026
10.3.1 GERMANY
10.3.1.1 Germany accounted for the largest share of the market in Europe in 2020
TABLE 69 GERMANY: KEY MACROINDICATORS
TABLE 70 GERMANY: MARKET, BY PRODUCT, 2019–2026
TABLE 71 GERMANY: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 72 GERMANY: THERAPEUTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 73 GERMANY: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 74 GERMANY: MARKET, BY USABILITY, 2019–2026
TABLE 75 GERMANY: MARKET, BY APPLICATION, 2019–2026
10.3.2 FRANCE
10.3.2.1 High incidences of neurological disorders drive the market growth in France
TABLE 76 FRANCE: KEY MACROINDICATORS
TABLE 77 FRANCE: MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 78 FRANCE: DIAGNOSTIC MARKET, BY PRODUCT, 2019–2026
TABLE 79 FRANCE: THERAPEUTIC MARKET, BY PRODUCT, 2019–2026
TABLE 80 FRANCE: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 81 FRANCE: MARKET, BY USABILITY, 2019–2026
TABLE 82 FRANCE: MARKET, BY APPLICATION, 2019–2026
10.3.3 UK
10.3.3.1 Increasing investments in public and private sectors to drive the market growth
TABLE 83 UK: KEY MACROINDICATORS
TABLE 84 UK: MARKET, BY PRODUCT, 2019–2026
TABLE 85 UK: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 86 UK: THERAPEUTIC MEDICAL ELECTRODES MARKET, BY PRODUCT, 2019–2026
TABLE 87 UK: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 88 UK: MARKET, BY USABILITY, 2019–2026
TABLE 89 UK: MARKET, BY APPLICATION, 2019–2026
10.3.4 REST OF EUROPE (ROE)
TABLE 90 ROE: HEALTHCARE EXPENDITURE, BY COUNTRY, 2010 VS. 2018 (% OF GDP)
TABLE 91 ROE: MARKET, BY PRODUCT, 2019–2026
TABLE 92 ROE: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 93 ROE: THERAPEUTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 94 ROE: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 95 ROE: MARKET, BY USABILITY, 2019–2026
TABLE 96 ROE: MARKET, BY APPLICATION, 2019–2026
10.4 ASIA PACIFIC
FIGURE 25 APAC: MARKET SNAPSHOT
TABLE 97 APAC: MARKET, BY COUNTRY, 2019–2026
TABLE 98 APAC: MARKET, BY PRODUCT, 2019–2026
TABLE 99 APAC: DIAGNOSTIC DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 100 APAC: THERAPEUTIC MEDICAL ELECTRODES MARKET, BY PRODUCT, 2019–2026
TABLE 101 APAC: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 102 APAC: MARKET, BY USABILITY, 2019–2026
TABLE 103 APAC: MARKET, BY APPLICATION, 2019–2026
10.4.1 JAPAN
10.4.1.1 The elderly population in Japan drives the market growth for medical devices
TABLE 104 JAPAN: KEY MACROINDICATORS
TABLE 105 JAPAN: MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 106 JAPAN: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 107 JAPAN: THERAPEUTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT,2019–2026
TABLE 108 JAPAN: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 109 JAPAN: MARKET, BY USABILITY, 2019–2026
TABLE 110 JAPAN: MARKET, BY APPLICATION, 2019–2026
10.4.2 CHINA
10.4.2.1 Developing healthcare infrastructure to boost the market growth for medical electrodes in China
TABLE 111 CHINA: KEY MACROINDICATORS
TABLE 112 CHINA: MARKET, BY PRODUCT, 2019–2026
TABLE 113 CHINA: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 114 CHINA: THERAPEUTIC MEDICAL ELECTRODES MARKET, BY PRODUCT, 2019–2026
TABLE 115 CHINA: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 116 CHINA: MARKET, BY USABILITY, 2019–2026
TABLE 117 CHINA: MARKET, BY APPLICATION, 2019–2026
10.4.3 INDIA
10.4.3.1 High incidence of cardiovascular and neurological diseases to drive the market
TABLE 118 INDIA: KEY MACROINDICATORS
TABLE 119 INDIA: MARKET, BY PRODUCT, 2019–2026
TABLE 120 INDIA: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 121 INDIA: THERAPEUTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 122 INDIA: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 123 INDIA: MARKET, BY USABILITY, 2019–2026
TABLE 124 INDIA: MARKET, BY APPLICATION, 2019–2026
10.4.4 ROAPAC
TABLE 125 ROAPAC: MARKET, BY PRODUCT, 2019–2026
TABLE 126 ROAPAC: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 127 ROAPAC: THERAPEUTIC MEDICAL ELECTRODES MARKET, BY PRODUCT, 2019–2026
TABLE 128 ROAPAC: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 129 ROAPAC: MARKET, BY USABILITY, 2019–2026
TABLE 130 ROAPAC: MARKET, BY APPLICATION, 2019–2026
10.5 REST OF THE WORLD
TABLE 131 ROW: MARKET, BY PRODUCT, 2019–2026
TABLE 132 ROW: DIAGNOSTIC MARKET for MEDICAL ELECTRODES, BY PRODUCT, 2019–2026
TABLE 133 ROW: THERAPEUTIC MEDICAL ELECTRODES INDUSTRY, BY PRODUCT, 2019–2026
TABLE 134 ROW: MARKET, BY TECHNOLOGY, 2019–2026
TABLE 135 ROW: MARKET, BY USABILITY,2019–2026
TABLE 136 ROW: MARKET, BY APPLICATION, 2019–2026
11 COMPETITIVE LANDSCAPE (Page No. - 139)
11.1 OVERVIEW
FIGURE 26 KEY DEVELOPMENTS IN THE MEDICAL ELECTRODES MARKET, JANUARY 2018–APRIL 2021
11.2 MARKET SHARE ANALYSIS
TABLE 137 GLOBAL MARKET: DEGREE OF COMPETITION (2019)
11.3 COMPANY EVALUATION QUADRANT
11.3.1 STARS
11.3.2 EMERGING LEADERS
11.3.3 PERVASIVE PLAYERS
11.3.4 PARTICIPANTS
FIGURE 27 GLOBAL MARKET: COMPANY EVALUATION QUADRANT, 2020
11.4 COMPANY EVALUATION QUADRANT: SMES/START-UPS
11.4.1 PROGRESSIVE COMPANIES
11.4.2 STARTING BLOCKS
11.4.3 RESPONSIVE COMPANIES
11.4.4 DYNAMIC COMPANIES
FIGURE 28 GLOBAL MARKET: COMPANY EVALUATION QUADRANT FOR SMES & START-UPS, 2020
11.5 COMPETITIVE SCENARIO
FIGURE 29 MARKET EVALUATION MATRIX, 2018–2021
11.5.1 DEALS
TABLE 138 DEALS
11.5.2 PRODUCT LAUNCHES
TABLE 139 PRODUCT LAUNCHES/APPROVALS
11.5.3 OTHER DEVELOPMENTS
TABLE 140 OTHER DEVELOPMENTS
11.6 COMPANY PRODUCT FOOTPRINT
TABLE 141 COMPANY FOOTPRINT
TABLE 142 COMPANY PRODUCT FOOTPRINT
TABLE 143 COMPANY GEOGRAPHICAL FOOTPRINT
12 COMPANY PROFILES (Page No. - 152)
(Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))*
12.1 KEY PLAYERS
12.1.1 CARDINAL HEALTH
FIGURE 30 CARDINAL HEALTH: COMPANY SNAPSHOT (2020)
12.1.2 3M
FIGURE 31 3M: COMPANY SNAPSHOT (2020)D
12.1.3 ZOLL MEDICAL CORPORATION (PART OF ASAHI KASEI CORPORATION)
FIGURE 32 ASAHI KASEI CORPORATION: COMPANY SNAPSHOT (2020)
12.1.4 MEDTRONIC
FIGURE 33 MEDTRONIC: COMPANY SNAPSHOT (2020)
12.1.5 AMBU A/S.
FIGURE 34 AMBU A/S: COMPANY SNAPSHOT (2020)
12.1.6 NATUS MEDICAL INCORPORATED
FIGURE 35 NATUS MEDICAL INCORPORATED: COMPANY SNAPSHOT (2020)
12.1.7 B. BRAUN MELSUNGEN AG
FIGURE 36 B. BRAUN MELSUNGEN AG: COMPANY SNAPSHOT (2020)
12.1.8 CONMED CORPORATION
FIGURE 37 CONMED CORPORATION: COMPANY SNAPSHOT (2020)
12.1.9 COMPUMEDICS LIMITED
FIGURE 38 COMPUMEDICS LIMITED.: COMPANY SNAPSHOT (2020)
12.1.10 COGNIONICS, INC.
12.1.11 GE HEALTHCARE (SUBSIDIARY OF GENERAL ELECTRIC COMPANY)
FIGURE 39 GENERAL ELECTRIC COMPANY: COMPANY SNAPSHOT (2020)
12.1.12 KONINKLIJKE PHILIPS N.V.
FIGURE 40 KONINKLIJKE PHILIPS N.V.: COMPANY SNAPSHOT (2020)
12.1.13 NIHON KOHDEN CORPORATION
FIGURE 41 NIHON KOHDEN CORPORATION.: COMPANY SNAPSHOT (2019)
12.1.14 RHYTHMLINK INTERNATIONAL, LLC
12.1.15 NISSHA MEDICAL TECHNOLOGIES (SUBSIDIARY OF NISSHA CO., LTD)
FIGURE 42 NISSHA CO., LTD.: COMPANY SNAPSHOT (2019)
12.2 OTHER PLAYERS
12.2.1 BOSTON SCIENTIFIC CORPORATION
12.2.2 COMEPA
12.2.3 EMED
12.2.4 G.TEC MEDICAL ENGINEERING GMBH
12.2.5 MEDICO ELECTRODES INTERNATIONAL LTD.
12.2.6 TZ MEDICAL
12.2.7 SOMNOMEDICSGMBH
12.2.8 R&D MEDICAL PRODUCTS
12.2.9 MEDLINE INDUSTRIES, INC.
12.2.10 WELLMMLEN HEALTHCARE TECH. (SUZHOU) CO., LTD.
*Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
13 APPENDIX (Page No. - 201)
13.1 DISCUSSION GUIDE
13.2 KNOWLEDGE STORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
13.3 AVAILABLE CUSTOMIZATIONS
13.4 RELATED REPORTS
13.5 AUTHOR DETAILS
This study involved four major activities in estimating the current size of the medical electrodes market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate the market size of segments and subsegments.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the medical electrodes market. The secondary sources used for this study include World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), National Center for Biotechnology Information (NCBI), World Federation of Neurology (WFN), American Brain Foundation, Neurology Asia Journal, Brain Injury Association of America (BIAA), American Neurological Association (ANA), American Heart Association (AHA), British Cardiac Patients Association (BCPA), European Journal of Neurology, Annual Reports, SEC Filings, Investor Presentations, Expert Interviews, and MarketsandMarkets Analysis. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information. The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the medical electrodes market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and market have been identified through extensive secondary research
- The revenues generated from the medical electrodes business of leading players have been determined through primary and secondary research
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
Data Triangulation
After arriving at the overall market size from the market size estimation process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Report Objectives
- To define, describe, and forecast the global medical electrodes market based on the product, usability, technology, application and region
- To provide detailed information regarding the major factors influencing the growth of the market (such as drivers and opportunities)
- To strategically analyze micromarkets with respect to individual growth trends, future prospects, and contributions to the overall medical electrodes market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five main regions, namely, North America, Europe, Asia Pacific, and the Rest of the World
- To strategically profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments such as acquisitions, product launches, expansions, agreements, partnerships, and R&D activities in the medical electrodes market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Company Information
- An additional five company profiles



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Medical Electrodes Market
What are the challenges faced by the key players in the global Medical Electrodes Market?
Which of the segments of Medical Electrodes Market is expected to grow at a highest CAGR during the forecast period?
How the technological innovations are fueling the revenue growth of the global Medical Electrodes Market?