Vaccines Market by Technology (Conjugate, Recombinant, Live Attenuated, Toxoid, Viral Vector, mRNA), Type (Monovalent, Multivalent), Disease (Pneumococcal, Flu, HPV, Herpes Zoster, MMR, Rotavirus, RSV), Route of Administration - Global Forecast to 2029
Market Growth Outlook Summary
The global vaccines market growth forecasted to transform from USD 78.0 billion in 2024 to USD 94.9 billion by 2029, driven by a CAGR of 4.0%. Similarly the vaccines market (excluding COVID -19 vaccines) is projected to reach USD 80.3 Billion by 2029 from USD 53.0 Billion in 2024, at a CAGR of 8.7% during the forecast period. Technological advancements in vaccine development, increasing government initiatives for immunization programs and expanding investments in research and development are some of the factors driving growth of this market.
Vaccines Market (Including Covid-19 Vaccines) Trends
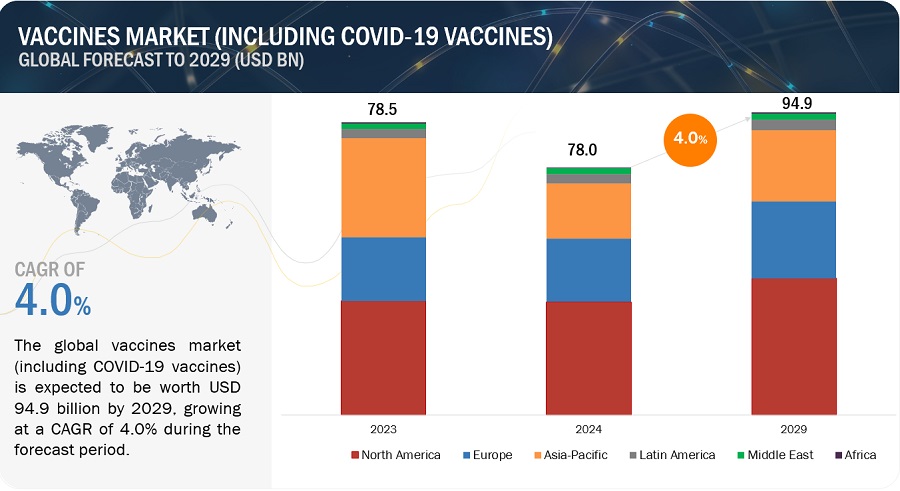
To know about the assumptions considered for the study, Request for Free Sample Report
Vaccines Market (Excluding Covid-19 Vaccines) Trends
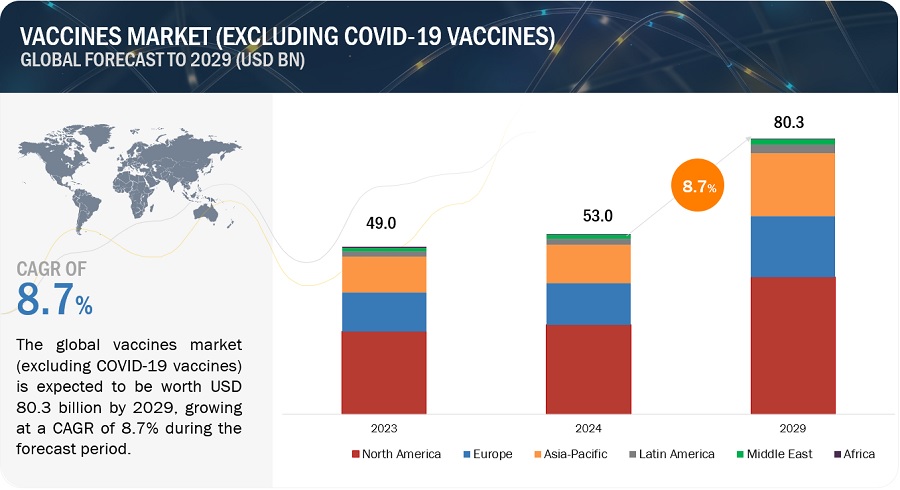
Vaccines Market Opportunities
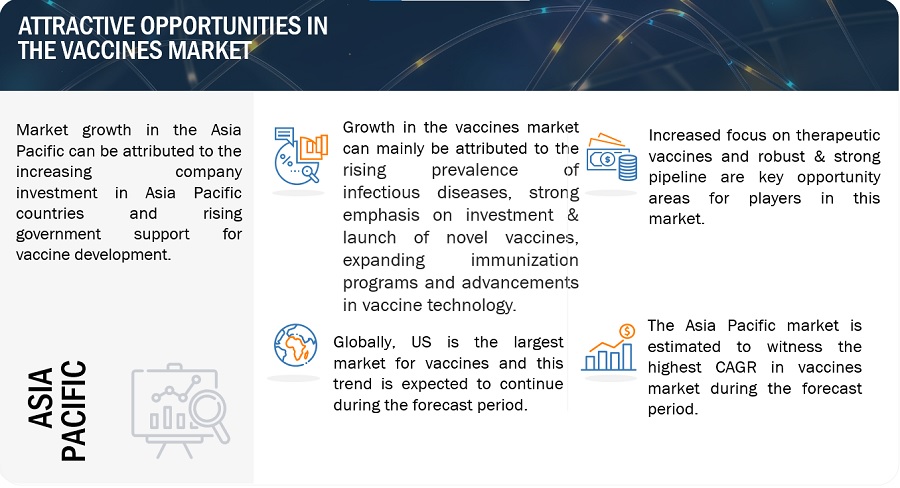
Vaccines market Dynamics
Driver: Focus on vaccine development and launches
The field of vaccine development has received increasing attention in recent years due to the complexity of products. Although this makes the development process challenging, it ensures that the resulting product is hard to duplicate. Due to this, market players have intensified research in this sector and focused on building their infrastructure. This factor is anticipated to have a positive impact on market growth. For instance, in 2023, Pfizer Inc (US) received approval from the US FDA for ABRYSVO, its bivalent RSV prefusion F (RSVpreF) vaccine to prevent LRTD and severe LRTD caused by RSV in infants from birth up to six months of age by active immunization of pregnant individuals at 32 through 36 weeks gestational age.
Restraint: High cost of vaccine development
Vaccine development is expensive, taking 10 to 15 years and costing between USD 800 million and USD 1 billion. Success is rare, making it tough for manufacturers to secure funds and cover operational expenses. Creating vaccines requires significant investment in R&D, production facilities, and specialized knowledge. Technology transfer depends on strong collaborations between partners, adding to the challenge of entering this field.
These hurdles create significant obstacles for new players in vaccine production. Consequently, there are fewer manufacturers in the vaccines segment than in other parts of the pharmaceutical industry. Moreover, the global vaccines industry is relatively small, accounting for less than 2% of the overall pharmaceutical market, which means fewer suppliers are attracted to it. For example, according to the United Nations Health Agency, as of September 2021, only 3% of people had been vaccinated with at least one dose of the COVID -19 vaccine, compared to 60.18% in high-income countries. According to Duke University’s Global Health Innovation Center, high-income countries have purchased more than half of the COVID -19 vaccine supply to date, and low-income countries, just 9% as of April 2021, thus ultimately close to getting vaccinated half of its population.
Opportunity: Rising focus on therapeutic vaccines
Advancements in immunology have opened doors to therapeutic vaccines, offering corrective treatments for various conditions rather than just prevention. These therapeutic vaccines address illnesses such as cancer, allergies, physiological disorders, and infectious diseases, benefiting a substantial patient base seeking alternative treatments. Scientists have actively explored and created innovative therapeutic vaccines for hypertension, dyslipidemia, Alzheimer’s, cancer, and inflammatory diseases, targeting specific self-antigens. If these vaccines prove as effective and safe as traditional medications, they could replace daily drug regimens for managing lifestyle-related diseases. For instance, in 2023, Pfizer received US FDA approval for ABRYSVO, which is a (Respiratory Syncytial Virus Vaccine), the company’s bivalent RSV prefusion F (RSVpreF) vaccine, to prevent lower respiratory tract disease caused by RSV in individuals 60 years and older.
Challenge: Stringent Regulatory Processes
Vaccine development requires identifying suitable antigens, adjuvants, and delivery methods while complying with stringent regulatory requirements. The complexity extends to the entire vaccine development process, from laboratory research to animal studies and clinical trials. Furthermore, differences in regulatory rules and procedures between developed and emerging economies have caused delays in vaccine approvals. Developing and emerging economies often have divergent regulatory requirements and processes. This variation can hinder the registration of vaccines, making it difficult for vaccine developers to navigate multiple sets of rules and standards.
Vaccines Market Ecosystem Analysis
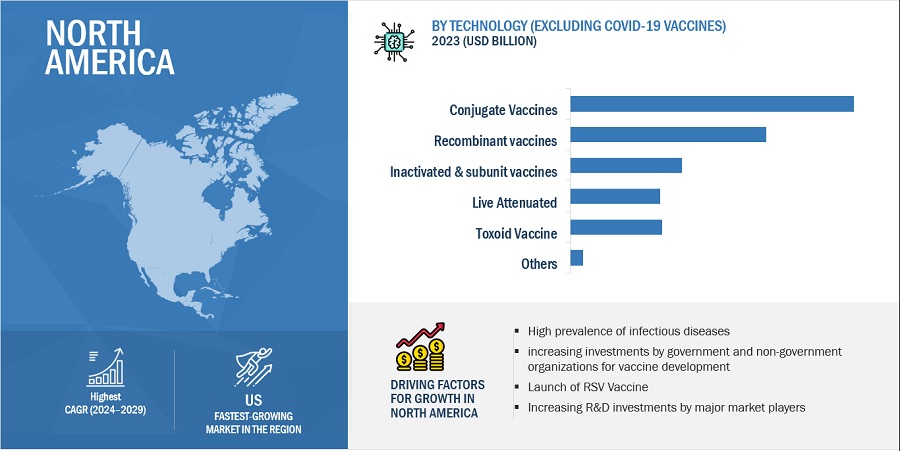
The conjugate vaccines segment is projected to hold the dominant position of vaccines market during the forecast period.
Based on technology, the market (excluding COVID -19 vaccines) is divided into various segments: conjugate vaccines, recombinant vaccines, inactivated & subunit vaccines, live attenuated vaccines, toxoid vaccines, and other vaccines. Conjugate vaccines effectively target challenging pathogens, preventing diseases such as meningitis and pneumonia this is factor is increasing adoption and demand for conjugate vaccines across the globe. Furthermore, expanding government immunization programs and rising awareness of preventive healthcare drive demand. Additionally, ongoing research and development efforts, strategic partnerships, and favorable regulatory environments support market expansion.
Multivalent vaccines segment accounted for dominant share of vaccines market during the forecast period.
Based on type, the market (excluding COVID-19 vaccines) is segmented into monovalent vaccines and multivalent vaccines. Multivalent vaccines segment accounted for dominant share in 2023. Multivalent vaccines offer convenience for healthcare providers and cost-effectiveness for healthcare systems by reducing the need for administering multiple separate vaccines. Furthermore, they contribute to herd immunity by increasing vaccination coverage across various target populations. Examples include combination vaccines like the measles, mumps, and rubella (MMR) vaccine and the pentavalent vaccine, which protects against diphtheria, tetanus, pertussis, Haemophilus influenzae type b (Hib), and hepatitis B.
HPV vaccine segment accounted for dominant share of vaccines market during the forecast period.
Based on disease indication, the market (excluding COVID-19 vaccines) is divided into pneumococcal disease, influenza, combination vaccines, HPV, meningococcal disease, herpes zoster, rotavirus, MMR, varicella, hepatitis, DTP, RSV, polio, and other disease indications. HPV vaccine segment accounted for dominant share in 2023. RSV disease indication segment is likely to grow at highest CAGR during the forecast period of 2024-2029. Launch of novel RSV vaccines and strong product pipeline are some of the factors uplifting the growth of the segment.
The intramuscular & subcutaneous segment accounted for the larger share of the vaccines market.
Based on route of administration, the market (excluding COVID-19 vaccines) is segmented into Intramuscular & subcutaneous, oral, and others. In 2023, the intramuscular & subcutaneous segment accounted for the larger share of the market. The dominance of intramuscular and subcutaneous routes of vaccine administration in the market is primarily due to their safety, effectiveness, and ease of administration.
Adult vaccine segment accounted for the dominant share of vaccines market during the forecast period.
Based on end user, the market (excluding COVID-19 vaccines) is segmented into pediatric vaccine and adult vaccine. In 2023, adult vaccine segment accounted for the domiant share. Launch of new RSV vaccine for adults, and aging population in many parts of the world has led to an increased focus on preventing vaccine-preventable diseases among adults are some of the factors uplifting the market growth.
North America accounted for the largest share of the vaccine market.
Based on the region, the market is segmented into North America, Europe, Asia Pacific, Latin America, the Middle East and Africa. In 2023, North America accounted for the largest share of the vaccine market (excluding COVID-19 vaccines), Europe is the second largest region in 2023. North America's dominance in the vaccine market can be attributed to several key factors such as advanced healthcare infrastructure and robust regulatory frameworks, facilitating the development, manufacturing, and distribution of vaccines. Furthermore, strong government support for vaccination programs and initiatives, coupled with favorable reimbursement policies, further bolster the demand for vaccines in North America. Asia Pacific region is anticipated to grow at a significant CAGR during the forecast period of 2024-2029.
Vaccines Market by Region
To know about the assumptions considered for the study, download the pdf brochure
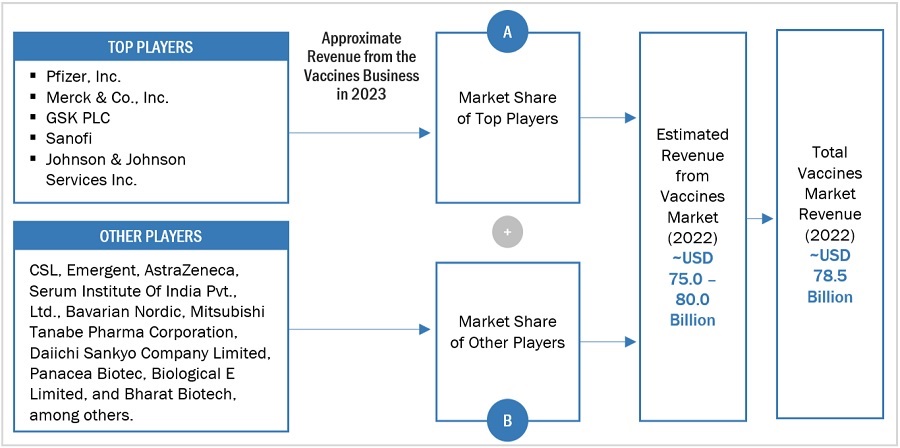
Key players in the vaccines market include GSK plc (UK), Merck & Co Inc (US), Pfizer Inc (US), Sanofi (France), CSL (Australia), Emergent (US), Johnson and Johnson Services, Inc. (US), Astrazeneca (UK), Serum Institute of India Pvt Ltd (India), Bavarian Nordic (Denmark), Mitsubishi Tanabe Pharma Corporation (Japan), Daiichi Sankyo Company, Limited. (Japan), Panacea Biotec (India), Biological E Ltd. (India), Bharat Biotech (India), Novavax (US), Inovio Pharmaceuticals (US), Sinovac (China), Incepta Pharma (Bangladesh), Valneva SE (France), VBI Vaccines Inc (US), Bio Farma (Indonesia), FSUE NPO MICROGEN (Russia), Zhi Fei Biological (China) and Indian Immunologicals Ltd. (India).
Vaccines Market Report Scope
|
Report Metric |
Details |
|
Market Size in 2024 |
USD 78.0 billion |
|
Forecasted Size by 2029 |
USD 94.9 billion |
|
Market Growth Rate |
Poised to Grow at a CAGR of 4.0% |
|
Market Driver |
Focus on vaccine development and launches |
|
Market Opportunity |
Rising focus on therapeutic vaccines |
This report categorizes the vaccines market to forecast revenue and analyze trends in each of the following submarkets:
By Technology
- Conjugate Vaccines
- Recombinant Vaccines
- Inactivated and Subunit Vaccines
- Live Attenuated Vaccines
- Toxoid Vaccines
- Other Vaccine Technologies
By Type
- Monovalent Vaccines
- Mulitvalent Vaccines
By Disease Indication
- Pneumococcal Disease
- Influenza
- Combination Vaccines
- HPV
- Meningococcal Disease
- Herpes Zoster
- Rotavirus
- MMR
- Varicella
- Hepatitis
- DTP
- Polio
- RSV
- Other Disease Indications
By Route of Administration
- Intramuscular & Subcutaneous
- Oral
- Other Route of Administration
By End User
- Pediatric Vaccine
- Adult Vaccine
By Region
- North America
- US
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe (ROE)
- Asia Pacific
- Japan
- South Korea
- China
- India
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- RoLATAM
- Middle East
- Africa
Recent Developments of Vaccines Market
- In April 2024, Bharat Biotech and Bilthoven Biologicals B.V. entered into a collaboration to produce and supply oral polio vaccines.
- In April 2024, VBI Vaccines Inc. expanded its stratergic collaboration with government of Canada to accelerate development proprietary MLE platform, enveloped virus-like particle vaccine technology that enables the coding of eVLPs using mRNA.
- In January 2024, Indian Immunologicals Ltd (IIL), launched India’s ‘first’ indigenously developed Hepatitis A vaccine, Havisure.
- In August 2023, Pfizer Inc., received US FDA approval for ABRYSVO (Respiratory Syncytial Virus Vaccine) for the prevention of LRTD and severe LRTD caused by RSV in infants from birth up to six months of age by active immunization of pregnant individuals at 32 through 36 weeks gestational age.
- In May 2023, GSK plc, received US FDA approval for Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older.
Frequently Asked Questions (FAQ):
What is the projected growth of the global vaccines market by 2029?
The global vaccines market is projected to grow from USD 78.0 billion in 2024 to USD 94.9 billion by 2029, demonstrating a robust CAGR of 4.0%.
What are the main factors driving the vaccines market?
The major factors driving the vaccines market include a growing focus on vaccine development, the launch of novel vaccines such as RSV vaccines, increasing awareness of preventive healthcare, and rising investments in R&D.
What are the challenges faced in the vaccine development process?
The key challenges include the high cost of vaccine development, which can take 10-15 years and cost between USD 800 million to USD 1 billion, as well as stringent regulatory processes that require compliance with varying rules across regions.
Which regions are expected to dominate the vaccines market?
North America is expected to dominate the vaccines market from 2024 to 2029, driven by favorable regulatory policies, a robust reimbursement scenario, and the presence of key market players. Europe is also expected to witness significant growth during this period.
What are the types of vaccines available in the global market?
The vaccines market includes a variety of types such as conjugate vaccines, recombinant vaccines, inactivated and subunit vaccines, live attenuated vaccines, toxoid vaccines, and others. These vaccines target various diseases, including pneumonia, meningitis, and hepatitis.
How do therapeutic vaccines present opportunities for market growth?
Therapeutic vaccines are gaining attention as they offer corrective treatments for conditions like cancer, allergies, and physiological disorders. These vaccines target specific self-antigens and could potentially replace daily medications for lifestyle-related diseases, driving future market growth.
What are the recent technological advancements in the vaccines market?
Recent technological advancements in the vaccines market include the development of mRNA vaccine platforms, which have demonstrated great potential during the COVID-19 pandemic. These innovations are expected to drive future vaccine development, especially in areas such as cancer and other infectious diseases.
What role do government initiatives play in expanding the vaccines market?
Government initiatives, such as expanded immunization programs and increased healthcare funding, play a critical role in boosting demand for vaccines. These efforts enhance accessibility to vaccines in both developed and developing regions, helping to protect against a wide range of diseases.
How is the increasing focus on preventive healthcare affecting the vaccines market?
The increasing focus on preventive healthcare is positively impacting the vaccines market by encouraging vaccination as a primary tool for disease prevention. Public awareness campaigns and healthcare policies aimed at reducing the burden of infectious diseases are driving market demand.
Which market segments are expected to grow the fastest in the vaccines industry?
The conjugate vaccines segment is expected to grow the fastest, driven by its effectiveness in preventing diseases like pneumonia and meningitis. Additionally, the recombinant vaccines segment is anticipated to see substantial growth due to advancements in genetic engineering technologies.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst


- 5.1 INTRODUCTION
-
5.2 MARKET DYNAMICSDRIVERS- Focus on vaccine development and launches- Rising prevalence of infectious diseases- Increasing immunization programs- Advancements in vaccine technology- Government support and funding for vaccine developmentRESTRAINTS- High cost of vaccine developmentOPPORTUNITIES- Rising focus on therapeutic vaccines- Extensive R&D for vaccines and increased investments in clinical trialsCHALLENGES- Stringent regulatory processes- Product recalls
-
5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES
-
5.4 PRICING ANALYSISAVERAGE SELLING PRICE OF PRODUCTS, BY KEY PLAYERAVERAGE SELLING PRICE, BY PRODUCT TYPEAVERAGE SELLING PRICE TREND
-
5.5 TECHNOLOGY ANALYSISMRNA VACCINESDNA TECHNOLOGYVLP VACCINE TECHNOLOGY
- 5.6 VALUE CHAIN ANALYSIS
-
5.7 PIPELINE ANALYSISKEY PIPELINE PRODUCTS
-
5.8 ECOSYSTEM/MARKET MAP
- 5.9 REGULATORY ANALYSIS
-
5.10 PORTER’S FIVE FORCES ANALYSISTHREAT OF NEW ENTRANTSTHREAT OF SUBSTITUTESBARGAINING POWER OF SUPPLIERSBARGAINING POWER OF BUYERSINTENSITY OF COMPETITIVE RIVALRY
-
5.11 PATENT ANALYSIS
- 5.12 KEY CONFERENCES & EVENTS, 2024–2025
-
5.13 KEY STAKEHOLDERS & BUYING CRITERIAKEY STAKEHOLDERS IN BUYING PROCESSKEY BUYING CRITERIA
- 6.1 INTRODUCTION
-
6.2 CONJUGATE VACCINESINCREASING PUBLIC-PRIVATE PARTNERSHIPS TO DRIVE MARKET
-
6.3 RECOMBINANT VACCINESLOW POST-VACCINATION REACTIONS AND REDUCED NEED FOR BOOSTER DOSES TO DRIVE MARKET
-
6.4 INACTIVATED & SUBUNIT VACCINESEASE OF STORAGE AND TRANSPORTATION TO SUPPORT GROWTH
-
6.5 LIVE ATTENUATED VACCINESHIGH COST OF STORAGE AND LIMITED FINANCIAL RESOURCES OF DISTRIBUTORS TO RESTRAIN MARKET
-
6.6 TOXOID VACCINESRISING PREVALENCE OF BACTERIAL INFECTIONS AMONG INFANTS AND CHILDREN TO DRIVE MARKET
-
6.7 VIRAL VECTOR VACCINESRISING INVESTMENT IN VACCINE DEVELOPMENT TO DRIVE MARKET
-
6.8 MRNA VACCINESINCREASING FOCUS ON MRNA VACCINE DEVELOPMENT TO DRIVE MARKET
- 6.9 OTHER VACCINES
- 7.1 INTRODUCTION
-
7.2 MULTIVALENT VACCINESINCREASED NEED FOR IMMUNIZATION AND COST-EFFECTIVENESS TO DRIVE MARKET
-
7.3 MONOVALENT VACCINESRISING R&D INVESTMENTS AND PREVALENCE OF INFECTIOUS DISEASES TO SUPPORT MARKET GROWTH
- 8.1 INTRODUCTION
-
8.2 PNEUMOCOCCAL DISEASEINCREASING INCIDENCE OF PNEUMONIA IN CHILDREN TO DRIVE MARKET
-
8.3 INFLUENZARISING NEED FOR IMMUNIZATION AGAINST VIRAL INFECTIONS TO DRIVE MARKET
-
8.4 COMBINATION VACCINESGROWING DEMAND FOR ALL-IN-ONE VACCINE TO DRIVE MARKET
-
8.5 HPVGROWING INCIDENCE OF SEXUALLY TRANSMITTED DISEASES TO DRIVE MARKET
-
8.6 MENINGOCOCCAL DISEASEINCREASING INITIATIVES BY GOVERNMENT AND NON-GOVERNMENT ORGANIZATIONS TO DRIVE MARKET
-
8.7 HERPES ZOSTERINCREASING INVESTMENT IN NOVEL VACCINE DEVELOPMENT TO DRIVE MARKET
-
8.8 ROTAVIRUSGROWING FOCUS OF GOVERNMENT AND NON-GOVERNMENT BODIES ON ROTAVIRUS PREVENTION TO PROPEL MARKET
-
8.9 MMRRISING INCIDENCE OF MEASLES, MUMPS, AND RUBELLA TO BOOST DEMAND
-
8.10 VARICELLAINCREASING PROMOTION OF IMMUNIZATION PROGRAMS TO SUPPORT MARKET GROWTH
-
8.11 HEPATITISLOW SOCIO-ECONOMIC STANDARDS OF LIVING AND HIGH CONTAMINATION IN DRINKING WATER TO DRIVE MARKET
-
8.12 DTPINCREASED OCCURRENCE OF DIPHTHERIA, TETANUS, AND PERTUSSIS IN EMERGING ECONOMIES TO DRIVE MARKET
-
8.13 POLIOINCREASING GOVERNMENT INITIATIVES AND IMMUNIZATION PROGRAMS TO DRIVE MARKET
-
8.14 RSVSTRONG PRODUCT PIPELINE AND NEW PRODUCT LAUNCHES TO PROPEL MARKET GROWTH
- 8.15 OTHER DISEASE INDICATIONS
- 9.1 INTRODUCTION
-
9.2 INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATIONEASE OF ABSORPTION AND BETTER IMMUNE RESPONSE TO DRIVE ADOPTION
-
9.3 ORAL ADMINISTRATIONREDUCED RISK OF BLOOD-TRANSMITTED INFECTIONS TO DRIVE ADOPTION
- 9.4 OTHER ROUTES OF ADMINISTRATION
- 10.1 INTRODUCTION
-
10.2 ADULT VACCINESADULT VACCINES TO COMMAND LARGER MARKET SHARE
-
10.3 PEDIATRIC VACCINESSUPPORT FROM GOVERNMENT AND NON-GOVERNMENT BODIES TO DRIVE MARKET
- 11.1 INTRODUCTION
-
11.2 NORTH AMERICANORTH AMERICA: RECESSION IMPACTUS- US to dominate North American marketCANADA- High incidence of infectious diseases to drive market
-
11.3 EUROPEEUROPE: RECESSION IMPACTGERMANY- Significant R&D investments and growing biotechnology industry to drive marketUK- Launch of new products and increased funding by government and non-government organizations to drive marketFRANCE- Favorable government initiatives for mass immunization to drive marketITALY- Higher investments by companies for increased production capacities to drive marketSPAIN- Rising investments in vaccine development by private organizations to drive marketREST OF EUROPE
-
11.4 ASIA PACIFICASIA PACIFIC: RECESSION IMPACTJAPAN- Favorable government initiatives to support market growthSOUTH KOREA- Strong government strategies for improved vaccine hubs to drive marketCHINA- China to hold largest share in APAC vaccines marketINDIA- Increasing government initiatives and development of new and improved vaccines to drive marketREST OF ASIA PACIFIC
-
11.5 LATIN AMERICALATIN AMERICA: RECESSION IMPACTBRAZIL- Rising focus on immunization programs to drive marketMEXICO- Trained workforce and ethnically varied population base for clinical trials to propel market growthREST OF LATIN AMERICA
-
11.6 MIDDLE EAST & AFRICAMIDDLE EAST & AFRICA: RECESSION IMPACTMIDDLE EAST- Increasing prevalence of infectious diseases to drive marketAFRICA- Availability of funds and grants from developed economies to drive market

- 12.1 INTRODUCTION
- 12.2 KEY STRATEGIES/RIGHT TO WIN
- 12.3 REVENUE SHARE ANALYSIS
- 12.4 MARKET SHARE ANALYSIS
-
12.5 COMPANY EVALUATION MATRIX: KEY PLAYERSSTARSEMERGING LEADERSPERVASIVE PLAYERSPARTICIPANTSCOMPANY FOOTPRINT
-
12.6 COMPANY EVALUATION MATRIX: START-UP/SME PLAYERSPROGRESSIVE COMPANIESRESPONSIVE COMPANIESDYNAMIC COMPANIESSTARTING BLOCKSCOMPETITIVE BENCHMARKING OF START-UPS/SMES
-
12.7 COMPETITIVE SCENARIOSPRODUCT LAUNCHES & APPROVALSDEALSEXPANSIONSOTHER DEVELOPMENTS
-
13.1 KEY PLAYERSGSK PLC- Business overview- Products offered- Recent developments- MnM viewMERCK & CO., INC.- Business overview- Products offered- Recent developments- MnM viewPFIZER INC.- Business overview- Products offered- Recent developments- MnM viewSANOFI- Business overview- Products offered- Recent developments- MnM viewCSL- Business overview- Products offered- Recent developments- MnM viewEMERGENT- Business overview- Products offered- Recent developmentsJOHNSON & JOHNSON SERVICES, INC.- Business overview- Products offered- Recent developmentsASTRAZENECA- Business overview- Products offered- Recent developmentsSERUM INSTITUTE OF INDIA PVT., LTD.- Business overview- Products offered- Recent developmentsBAVARIAN NORDIC- Business overview- Products offered- Recent developmentsMITSUBISHI TANABE PHARMA CORPORATION- Business overview- Products offered- Recent developmentsDAIICHI SANKYO COMPANY, LIMITED- Business overview- Products offered- Recent developmentsPANACEA BIOTEC- Business overview- Products offered- Recent developments- Other developmentsBIOLOGICAL E LIMITED- Business overview- Products offered- Recent developmentsBHARAT BIOTECH- Business overview- Products offered- Recent developmentsNOVAVAX- Business overview- Products offered- Recent developmentsINOVIO PHARMACEUTICALS- Business overview- Products offered- Recent developments
-
13.2 OTHER PLAYERSSINOVACINCEPTA PHARMACEUTICALS LTD.VALNEVA SEVBI VACCINE INC.BIO FARMAMICROGENZHI FEI BIOLOGICALINDIAN IMMUNOLOGICALS LIMITED
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
- 14.3 CUSTOMIZATION OPTIONS
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS
- TABLE 1 IMPACT ANALYSIS: VACCINES MARKET
- TABLE 2 NIH FUNDING FOR VACCINE RESEARCH, 2020–2024 (USD MILLION)
- TABLE 3 AVERAGE SELLING PRICE OF PEDIATRIC VACCINES
- TABLE 4 AVERAGE SELLING PRICE OF ADULT VACCINES
- TABLE 5 AVERAGE SELLING PRICE OF CONJUGATE VACCINES
- TABLE 6 AVERAGE SELLING PRICE OF RECOMBINANT VACCINES
- TABLE 7 AVERAGE SELLING PRICE OF INACTIVATED & SUBUNIT VACCINES
- TABLE 8 AVERAGE SELLING PRICE OF LIVE ATTENUATED VACCINES
- TABLE 9 AVERAGE SELLING PRICE OF TOXOID VACCINES
- TABLE 10 AVERAGE SELLING PRICE OF M-RNA VACCINES
- TABLE 11 PIPELINE PRODUCTS UNDER PHASE 2 AND PHASE 3 CLINICAL TRIALS
- TABLE 12 VACCINE PIPELINE PRODUCTS UNDER PHASE-3 CLINICAL TRIALS FOR RSV
- TABLE 13 KEY PIPELINE VACCINES: GSK PLC
- TABLE 14 KEY PIPELINE VACCINES: MERCK & CO., INC.
- TABLE 15 KEY PIPELINE VACCINES: PFIZER INC.
- TABLE 16 KEY PIPELINE VACCINES: SANOFI S.A.
- TABLE 17 ROLE IN ECOSYSTEM: VACCINES MARKET
- TABLE 18 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 19 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 20 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 21 LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 22 MIDDLE EAST: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 23 AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 24 PORTER’S FIVE FORCES ANALYSIS
- TABLE 25 VACCINES MARKET: INDICATIVE LIST OF PATENTS
- TABLE 26 DETAILED LIST OF CONFERENCES & EVENTS
- TABLE 27 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 28 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 29 CONJUGATE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 30 NORTH AMERICA: CONJUGATE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 31 EUROPE: CONJUGATE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 32 ASIA PACIFIC: CONJUGATE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 33 LATIN AMERICA: CONJUGATE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 34 EXAMPLES OF RECOMBINANT VACCINES
- TABLE 35 RECOMBINANT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 36 NORTH AMERICA: RECOMBINANT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 37 EUROPE: RECOMBINANT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 38 ASIA PACIFIC: RECOMBINANT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 39 LATIN AMERICA: RECOMBINANT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 40 EXAMPLES OF INACTIVATED & SUBUNIT VACCINES
- TABLE 41 INACTIVATED & SUBUNIT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 42 NORTH AMERICA: INACTIVATED & SUBUNIT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 43 EUROPE: INACTIVATED & SUBUNIT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 44 ASIA PACIFIC: INACTIVATED & SUBUNIT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 45 LATIN AMERICA: INACTIVATED & SUBUNIT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 46 EXAMPLES OF LIVE ATTENUATED VACCINES
- TABLE 47 LIVE ATTENUATED VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 48 NORTH AMERICA: LIVE ATTENUATED VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 49 EUROPE: LIVE ATTENUATED VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 50 ASIA PACIFIC: LIVE ATTENUATED VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 51 LATIN AMERICA: LIVE ATTENUATED VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 52 EXAMPLES OF TOXOID VACCINES
- TABLE 53 TOXOID VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 54 NORTH AMERICA: TOXOID VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 55 EUROPE: TOXOID VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 56 ASIA PACIFIC: TOXOID VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 57 LATIN AMERICA: TOXOID VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 58 VIRAL VECTOR VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 59 NORTH AMERICA: VIRAL VECTOR VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 60 EUROPE: VIRAL VECTOR VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 61 ASIA PACIFIC: VIRAL VECTOR VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 62 LATIN AMERICA: VIRAL VECTOR VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 63 MRNA VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 64 NORTH AMERICA: MRNA VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 65 EUROPE: MRNA VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 66 ASIA PACIFIC: MRNA VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 67 LATIN AMERICA: MRNA VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 68 OTHER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 69 NORTH AMERICA: OTHER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 70 EUROPE: OTHER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 71 ASIA PACIFIC: OTHER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 72 LATIN AMERICA: OTHER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 73 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 74 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 75 EXAMPLES OF MULTIVALENT VACCINES
- TABLE 76 MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 77 NORTH AMERICA: MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 78 EUROPE: MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 79 ASIA PACIFIC: MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 80 LATIN AMERICA: MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 81 EXAMPLES OF MONOVALENT VACCINES
- TABLE 82 MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 83 NORTH AMERICA: MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 84 EUROPE: MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 85 ASIA PACIFIC: MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 86 LATIN AMERICA: MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 87 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 88 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 89 LIST OF COMMERCIALLY AVAILABLE PNEUMOCOCCAL DISEASE VACCINES
- TABLE 90 PNEUMOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 91 NORTH AMERICA: PNEUMOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 92 EUROPE: PNEUMOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 93 ASIA PACIFIC: PNEUMOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 94 LATIN AMERICA: PNEUMOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 95 LIST OF COMMERCIALLY AVAILABLE INFLUENZA VACCINES
- TABLE 96 INFLUENZA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 97 NORTH AMERICA: INFLUENZA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 98 EUROPE: INFLUENZA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 99 ASIA PACIFIC: INFLUENZA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 100 LATIN AMERICA: INFLUENZA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 101 LIST OF COMMERCIALLY AVAILABLE COMBINATION VACCINES
- TABLE 102 COMBINATION VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 103 NORTH AMERICA: COMBINATION VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 104 EUROPE: COMBINATION VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 105 ASIA PACIFIC: COMBINATION VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 106 LATIN AMERICA: COMBINATION VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 107 LIST OF COMMERCIALLY AVAILABLE HPV VACCINES
- TABLE 108 HPV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 109 NORTH AMERICA: HPV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 110 EUROPE: HPV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 111 ASIA PACIFIC: HPV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 112 LATIN AMERICA: HPV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 113 LIST OF COMMERCIALLY AVAILABLE MENINGOCOCCAL DISEASE VACCINES
- TABLE 114 MENINGOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 115 NORTH AMERICA: MENINGOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 116 EUROPE: MENINGOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 117 ASIA PACIFIC: MENINGOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 118 LATIN AMERICA: MENINGOCOCCAL DISEASE VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 119 HERPES ZOSTER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 120 NORTH AMERICA: HERPES ZOSTER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 121 EUROPE: HERPES ZOSTER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 122 ASIA PACIFIC: HERPES ZOSTER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 123 LATIN AMERICA: HERPES ZOSTER VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 124 LIST OF COMMERCIALLY AVAILABLE ROTAVIRUS VACCINES
- TABLE 125 ROTAVIRUS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 126 NORTH AMERICA: ROTAVIRUS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 127 EUROPE: ROTAVIRUS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 128 ASIA PACIFIC: ROTAVIRUS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 129 LATIN AMERICA: ROTAVIRUS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 130 LIST OF COMMERCIALLY AVAILABLE MMR VACCINES
- TABLE 131 MMR VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 132 NORTH AMERICA: MMR VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 133 EUROPE: MMR VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 134 ASIA PACIFIC: MMR VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 135 LATIN AMERICA: MMR VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 136 LIST OF COMMERCIALLY AVAILABLE VARICELLA VACCINES
- TABLE 137 VARICELLA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 138 NORTH AMERICA: VARICELLA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 139 EUROPE: VARICELLA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 140 ASIA PACIFIC: VARICELLA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 141 LATIN AMERICA: VARICELLA VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 142 LIST OF COMMERCIALLY AVAILABLE HEPATITIS VACCINES
- TABLE 143 HEPATITIS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 144 NORTH AMERICA: HEPATITIS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 145 EUROPE: HEPATITIS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 146 ASIA PACIFIC: HEPATITIS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 147 LATIN AMERICA: HEPATITIS VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 148 LIST OF COMMERCIALLY AVAILABLE DTP VACCINES
- TABLE 149 DTP VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 150 NORTH AMERICA: DTP VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 151 EUROPE: DTP VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 152 ASIA PACIFIC: DTP VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 153 LATIN AMERICA: DTP VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 154 POLIO VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 155 NORTH AMERICA: POLIO VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 156 EUROPE: POLIO VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 157 ASIA PACIFIC: POLIO VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 158 LATIN AMERICA: POLIO VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 159 RSV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 160 NORTH AMERICA: RSV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 161 EUROPE: RSV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 162 ASIA PACIFIC: RSV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 163 LATIN AMERICA: RSV VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 164 LIST OF COMMERCIALLY AVAILABLE VACCINES FOR OTHER DISEASE INDICATIONS
- TABLE 165 VACCINES MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 166 VACCINES MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 167 NORTH AMERICA: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 168 NORTH AMERICA: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 169 EUROPE: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 170 EUROPE: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 171 ASIA PACIFIC: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 172 ASIA PACIFIC: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 173 LATIN AMERICA: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 174 LATIN AMERICA: VACCINES MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 175 VACCINES MARKET, BY ROUTE OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), 2022–2029 (USD MILLION)
- TABLE 176 VACCINES MARKET, BY ROUTE OF ADMINISTRATION (INCLUDING COVID-19 VACCINES), 2022–2029 (USD MILLION)
- TABLE 177 ADVANTAGES AND DISADVANTAGES OF INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION
- TABLE 178 VACCINES MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 179 NORTH AMERICA: VACCINES MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 180 EUROPE: VACCINES MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 181 ASIA PACIFIC: VACCINES MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 182 LATIN AMERICA: VACCINES MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 183 VACCINES MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 184 NORTH AMERICA: VACCINES MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 185 EUROPE: VACCINES MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 186 ASIA PACIFIC: VACCINES MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 187 LATIN AMERICA: VACCINES MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 188 VACCINES MARKET FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 189 NORTH AMERICA: VACCINES MARKET FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 190 EUROPE: VACCINES MARKET FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 191 ASIA PACIFIC: VACCINES MARKET FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 192 LATIN AMERICA: VACCINES MARKET FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 193 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 194 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 195 ADULT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 196 NORTH AMERICA: ADULT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 197 EUROPE: ADULT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 198 ASIA PACIFIC: ADULT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 199 LATIN AMERICA: ADULT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 200 PEDIATRIC VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 201 NORTH AMERICA: PEDIATRIC VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 202 EUROPE: PEDIATRIC VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 203 ASIA PACIFIC: PEDIATRIC VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 204 LATIN AMERICA: PEDIATRIC VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 205 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 206 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
- TABLE 207 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 208 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 209 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 210 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 211 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 212 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 213 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 214 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 215 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 216 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 217 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 218 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 219 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 220 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 221 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 222 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 223 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 224 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 225 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 226 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 227 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 228 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 229 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 230 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 231 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 232 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 233 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 234 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 235 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 236 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 237 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 238 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 239 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 240 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 241 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 242 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 243 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 244 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 245 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 246 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 247 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 248 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 249 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 250 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 251 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 252 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 253 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 254 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 255 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 256 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 257 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 258 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 259 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 260 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 261 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 262 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 263 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 264 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 265 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 266 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 267 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 268 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 269 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 270 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 271 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 272 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 273 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 274 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 275 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 276 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 277 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 278 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 279 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 280 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 281 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 282 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 283 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 284 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 285 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 286 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 287 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 288 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 289 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 290 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 291 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 292 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 293 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 294 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 295 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 296 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 297 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 298 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 299 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
- TABLE 300 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 301 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 302 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
- TABLE 303 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
- TABLE 304 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
- TABLE 305 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
- TABLE 306 VACCINES MARKET: DEGREE OF COMPETITION
- TABLE 307 TYPE FOOTPRINT
- TABLE 308 REGIONAL FOOTPRINT
- TABLE 309 VACCINES MARKET: DETAILED LIST OF KEY START-UPS/SMES
- TABLE 310 VACCINES MARKET: COMPETITIVE BENCHMARKING OF START-UPS/SMES
- TABLE 311 VACCINES MARKET: PRODUCT LAUNCHES & APPROVALS (JANUARY 2021−APRIL 2024)
- TABLE 312 VACCINES MARKET: DEALS (JANUARY 2021−APRIL 2024)
- TABLE 313 VACCINES MARKET: EXPANSIONS (JANUARY 2021−APRIL 2024)
- TABLE 314 VACCINES MARKET: OTHER DEVELOPMENTS (JANUARY 2021−APRIL 2024)
- TABLE 315 GSK PLC: COMPANY OVERVIEW
- TABLE 316 GSK PLC: PRODUCTS OFFERED
- TABLE 317 GSK PLC: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 318 GSK PLC: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 319 GSK PLC: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 320 MERCK & CO., INC.: COMPANY OVERVIEW
- TABLE 321 MERCK & CO., INC.: PRODUCTS OFFERED
- TABLE 322 MERCK & CO., INC.: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 323 MERCK & CO., INC.: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 324 MERCK & CO., INC.: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 325 PFIZER INC.: COMPANY OVERVIEW
- TABLE 326 PFIZER INC.: PRODUCTS OFFERED
- TABLE 327 PFIZER INC.: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 328 PFIZER INC.: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 329 PFIZER INC.: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 330 SANOFI: COMPANY OVERVIEW
- TABLE 331 SANOFI: PRODUCTS OFFERED
- TABLE 332 SANOFI: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 333 SANOFI: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 334 SANOFI: EXPANSIONS (JANUARY 2020–MARCH 2024)
- TABLE 335 SANOFI: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 336 CSL: COMPANY OVERVIEW
- TABLE 337 CSL: PRODUCTS OFFERED
- TABLE 338 CSL: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 339 CSL: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 340 CSL: EXPANSIONS (JANUARY 2020–MARCH 2024)
- TABLE 341 CSL: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 342 EMERGENT: COMPANY OVERVIEW
- TABLE 343 EMERGENT: PRODUCTS OFFERED
- TABLE 344 EMERGENT: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 345 EMERGENT: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 346 EMERGENT: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 347 JOHNSON & JOHNSON SERVICES INC.: COMPANY OVERVIEW
- TABLE 348 JOHNSON & JOHNSON SERVICES INC.: PRODUCTS OFFERED
- TABLE 349 JOHNSON & JOHNSON SERVICES INC.: PRODUCT APPROVALS (JANUARY 2020− MARCH 2024)
- TABLE 350 JOHNSON & JOHNSON SERVICES INC.: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 351 ASTRAZENECA: COMPANY OVERVIEW
- TABLE 352 ASTRAZENECA: PRODUCTS OFFERED
- TABLE 353 ASTRAZENECA: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 354 ASTRAZENECA: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 355 ASTRAZENECA: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 356 SERUM INSTITUTE OF INDIA PVT., LTD.: COMPANY OVERVIEW
- TABLE 357 SERUM INSTITUTE OF INDIA PVT., LTD.: PRODUCTS OFFERED (SUPPLIED OVERSEAS)
- TABLE 358 SERUM INSTITUTE OF INDIA PVT., LTD.: PRODUCTS OFFERED (SUPPLIED IN INDIA)
- TABLE 359 SERUM INSTITUTE OF INDIA PVT., LTD.: PRODUCTS LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 360 SERUM INSTITUTE OF INDIA PVT., LTD.: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 361 BAVARIAN NORDIC: COMPANY OVERVIEW
- TABLE 362 BAVARIAN NORDIC: PRODUCTS OFFERED
- TABLE 363 BAVARIAN NORDIC: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 364 BAVARIAN NORDIC: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 365 BAVARIAN NORDIC: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 366 MITSUBISHI TANABE PHARMA CORPORATION: COMPANY OVERVIEW
- TABLE 367 MITSUBISHI TANABE PHARMA CORPORATION: PRODUCTS OFFERED
- TABLE 368 MITSUBISHI TANABE PHARMA CORPORATION: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 369 MITSUBISHI TANABE PHARMA CORPORATION: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 370 DAIICHI SANKYO COMPANY, LIMITED: COMPANY OVERVIEW
- TABLE 371 DAIICHI SANKYO COMPANY, LIMITED: PRODUCTS OFFERED
- TABLE 372 DAIICHI SANKYO COMPANY, LIMITED: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 373 PANACEA BIOTEC: COMPANY OVERVIEW
- TABLE 374 PANACEA BIOTEC: PRODUCTS OFFERED
- TABLE 375 PANACEA BIOTEC: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 376 PANACEA BIOTEC: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 377 BIOLOGICAL E LIMITED: COMPANY OVERVIEW
- TABLE 378 BIOLOGICAL E LIMITED: PRODUCTS OFFERED (INDIAN MARKET)
- TABLE 379 BIOLOGICAL E LIMITED: PRODUCTS OFFERED (INTERNATIONAL MARKET)
- TABLE 380 BIOLOGICAL E LIMITED: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 381 BIOLOGICAL E LIMITED: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 382 BIOLOGICAL E LIMITED: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- TABLE 383 BHARAT BIOTECH: COMPANY OVERVIEW
- TABLE 384 BHARAT BIOTECH: PRODUCTS OFFERED (VACCINES)
- TABLE 385 BHARAT BIOTECH: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 386 BHARAT BIOTECH: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 387 NOVAVAX: COMPANY OVERVIEW
- TABLE 388 NOVAVAX: PRODUCTS OFFERED (VACCINES)
- TABLE 389 NOVAVAX: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
- TABLE 390 NOVAVAX: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 391 INOVIO PHARMACEUTICALS: COMPANY OVERVIEW
- TABLE 392 INOVIO PHARMACEUTICALS: PRODUCTS OFFERED (VACCINES)
- TABLE 393 INOVIO PHARMACEUTICALS: DEALS (JANUARY 2020–MARCH 2024)
- TABLE 394 INOVIO PHARMACEUTICALS: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
- FIGURE 1 RESEARCH DESIGN
- FIGURE 2 BREAKDOWN OF PRIMARIES
- FIGURE 3 MARKET SIZE ESTIMATION (SUPPLY SIDE ANALYSIS), 2023
- FIGURE 4 MARKET SIZE ESTIMATION: APPROACH 1 (REVENUE SHARE ANALYSIS), 2023
- FIGURE 5 ILLUSTRATIVE EXAMPLE OF PFIZER INC.: REVENUE SHARE ANALYSIS, 2023
- FIGURE 6 MARKET VALIDATION FROM PRIMARY EXPERTS
- FIGURE 7 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
- FIGURE 8 VACCINES MARKET: CAGR PROJECTION (2024-2029)
- FIGURE 9 VACCINES MARKET: GROWTH ANALYSIS OF DRIVERS, RESTRAINTS, CHALLENGES, AND OPPORTUNITIES
- FIGURE 10 DATA TRIANGULATION METHODOLOGY
- FIGURE 11 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2024 VS. 2029 (USD MILLION)
- FIGURE 12 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2024 VS. 2029 (USD MILLION)
- FIGURE 13 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2024 VS. 2029 (USD MILLION)
- FIGURE 14 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY TYPE, 2024 VS. 2029 (USD MILLION)
- FIGURE 15 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2024 VS. 2029 (USD MILLION)
- FIGURE 16 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2024 VS. 2029 (USD MILLION)
- FIGURE 17 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2024 VS. 2029 (USD MILLION)
- FIGURE 18 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2024 VS. 2029 (USD MILLION)
- FIGURE 19 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2024 VS. 2029 (USD MILLION)
- FIGURE 20 VACCINES MARKET (INCLUDING COVID-19 VACCINES), BY END USER, 2024 VS. 2029 (USD MILLION)
- FIGURE 21 GEOGRAPHICAL SNAPSHOT OF VACCINES MARKET (EXCLUDING COVID-19 VACCINES)
- FIGURE 22 GEOGRAPHICAL SNAPSHOT OF VACCINES MARKET (INCLUDING COVID-19 VACCINES)
- FIGURE 23 RISING PREVALENCE OF INFECTIOUS DISEASES TO DRIVE MARKET
- FIGURE 24 LAUNCH OF RSV VACCINES AND STRONG PRODUCT PIPELINE TO DRIVE MARKET
- FIGURE 25 US AND CONJUGATE VACCINES ACCOUNTED FOR LARGEST SHARE OF NORTH AMERICAN VACCINES MARKET IN 2023
- FIGURE 26 ASIA PACIFIC MARKETS TO REGISTER HIGHEST GROWTH RATES DURING FORECAST PERIOD
- FIGURE 27 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES: VACCINES MARKET
- FIGURE 28 US: INCIDENCE OF TUBERCULOSIS, 2017–2021
- FIGURE 29 REVENUE SHIFTS AND NEW REVENUE POCKETS FOR VACCINE PROVIDERS
- FIGURE 30 VALUE CHAIN ANALYSIS: RAW MATERIAL AND MANUFACTURING PHASES TO CONTRIBUTE MAXIMUM VALUE
- FIGURE 31 VACCINES MARKET: CLINICAL TRIALS, BY PHASE
- FIGURE 32 VACCINES MARKET: CLINICAL TRIALS, BY DISEASE INDICATION
- FIGURE 33 ECOSYSTEM/MARKET MAP
- FIGURE 34 PATENT APPLICATIONS FOR VACCINES, SEPTEMBER 2013–SEPTEMBER 2023
- FIGURE 35 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF VACCINES
- FIGURE 36 KEY BUYING CRITERIA FOR END USERS
- FIGURE 37 US: NUMBER OF COVID-19 CASES, 2020–2023
- FIGURE 38 NORTH AMERICA: VACCINES MARKET SNAPSHOT (EXCLUDING COVID-19 VACCINES)
- FIGURE 39 ASIA PACIFIC: VACCINES MARKET SNAPSHOT (EXCLUDING COVID-19 VACCINES)
- FIGURE 40 STRATEGIES ADOPTED BY MAJOR PLAYERS IN VACCINES MARKET
- FIGURE 41 REVENUE SHARE ANALYSIS, 2021–2023
- FIGURE 42 MARKET SHARE ANALYSIS OF KEY PLAYERS (2023)
- FIGURE 43 VACCINES MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
- FIGURE 44 VACCINES MARKET: COMPANY EVALUATION MATRIX (START-UP/ SME PLAYERS), 2023
- FIGURE 45 GSK PLC: COMPANY SNAPSHOT (2023)
- FIGURE 46 MERCK & CO., INC.: COMPANY SNAPSHOT (2023)
- FIGURE 47 PFIZER INC.: COMPANY SNAPSHOT (2023)
- FIGURE 48 SANOFI: COMPANY SNAPSHOT (2023)
- FIGURE 49 CSL: COMPANY SNAPSHOT (2023)
- FIGURE 50 EMERGENT: COMPANY SNAPSHOT (2023)
- FIGURE 51 JOHNSON & JOHNSON SERVICES INC.: COMPANY SNAPSHOT (2023)
- FIGURE 52 ASTRAZENECA: COMPANY SNAPSHOT (2023)
- FIGURE 53 BAVARIAN NORDIC: COMPANY SNAPSHOT (2023)
- FIGURE 54 DAIICHI SANKYO COMPANY, LIMITED: COMPANY SNAPSHOT (2022)
- FIGURE 55 PANACEA BIOTEC: COMPANY SNAPSHOT (2022)
- FIGURE 56 NOVAVAX: COMPANY SNAPSHOT (2023)
- FIGURE 57 INOVIO PHARMACEUTICALS: COMPANY SNAPSHOT (2023)
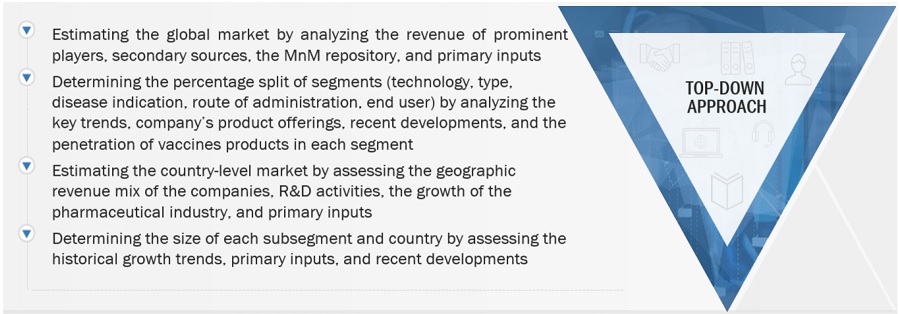
This study involved four major activities in estimating the current size of the vaccines market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate the market size of segments and subsegments.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the vaccines market. The secondary sources used for this study include World Health Organization (WHO), the Organization for Economic Co-operation and Development (OECD), National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), the Global Cancer Observatory (GLOBOCAN), the National Institutes of Health (NIH), Center of Disease Control & Prevention (CDC), US Department of Health and Human Services, National Institutes of Health (NIH), National Library of Medicine, National Center for Biotechnology Information (NCBI), National Institute of Allergy and Infectious Diseases (NIAID), World Cancer Research Fund International (WCRF International), European Medicines Agency (EMA), The National Medical Products Administration (NMPA), Global Alliance for Vaccines and Immunization (GAVI), United States Food & Drug Administration (US FDA), Orange book, Purple book, Clinical trials.gov, Pan American Health Organization (PAHO), United Nation International Children’s Emergency Fund (UNICEF), Department of health and Human Services (HHS), International Society for Vaccines (ISV). The Central Drugs Standard Control Organization (CDSCO); ACS Journals; Corporate filings such as annual reports, SEC filings, investor presentations, and financial statements; research journals; press releases; and trade, business, and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the global vaccines market, which was validated through primary research.
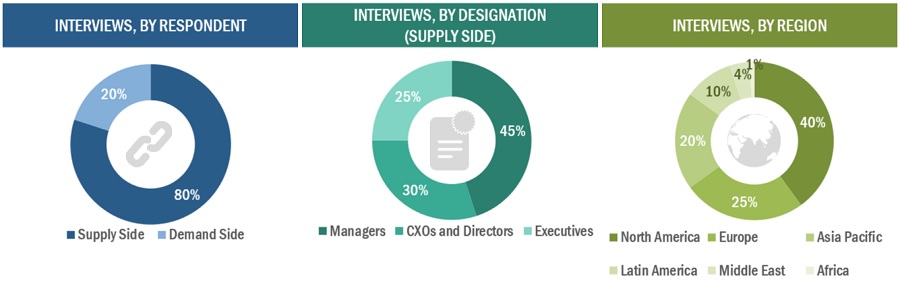
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information. The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The global size of the vaccines market was estimated through multiple approaches. A detailed market estimation approach was followed to estimate and validate the value of the market and other dependent submarkets. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The major players in the industry and market have been identified through extensive primary and secondary research.
- The revenues generated from the vaccines service business of players operating in the market have been determined through secondary research and primary analysis.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
- Pipeline Analysis is conducted for various disease indications for forecasting the market.
Global Vaccines Market Size: Bottom-Up Approach
To know about the assumptions considered for the study, Request for Free Sample Report
Top-down Approach-
Data Triangulation
After estimating the overall market size from the market size estimation process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
A vaccine is a biologically formulated product engineered to elicit active acquired immunity against a specific infectious or malignant disease. Vaccines operate by stimulating the immune system to detect and combat harmful agents, such as viruses or bacteria. They usually consist of components that resemble the disease-causing microorganism, often in weakened or deactivated forms, along with their toxins or surface proteins. The report exclusively encompasses human vaccines and does not cover any veterinary vaccines which has been excluded from the scope of our study.
Key Stakeholders
- Vaccine product manufacturers and suppliers
- Distributors and suppliers of vaccine products
- Vaccine research institutes
- Biotechnology and biopharmaceutical companies
- Contract manufacturing organizations (CMOs)
- Contract development and manufacturing organizations (CDMO)
- Suppliers and distributors of pharmaceutical products
- Research and development (R&D) companies
- Drug Manufacturers, Vendors, and Distributors
- Immunization centres
- Hospitals and laboratories
- Trade associations and industry bodies
- Regulatory bodies and government organizations
- Venture capitalists and investors
- Hospitals
- Specialty Clinics
Report Objectives
- To define, describe, and forecast the vaccines market based on technology, type, disease indication, route of administration, end user, and region.
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall vaccines market.
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders.
- To forecast the size of the market segments with respect to five regions, namely: North America, Europe, the Asia Pacific (APAC), Latin America (LATAM), Middle East and Africa.
- To profile the key players and analyze their market shares and core competencies2
- To track and analyze competitive developments, such as acquisitions, product launches, expansions, regulatory approvals, agreements, partnerships, and collaborations.
- To provide information on the pipelines analysis of vaccines undergoing phase 2 and phase 3 clinical trials.
- To benchmark players within the market using the proprietary “Competitive Leadership Mapping” framework, which analyzes market players on various parameters within the broad categories of business and product excellence strategy
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Product Analysis
- Product matrix, which provides a detailed comparison of the product portfolio of each company in the vaccines Market



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Vaccines Market
What are the growing opportunities in Vaccines Market Size, Share, Growth, Covid-19 Impact Analysis, forecasts to 2028 ?
Which are some of the significant growth strategies adopted by leading companies in this market?
According to this report which are the top 3 companies operating in the global vaccine market?
How the Asia Pacific region in the Global Vaccines Market is Expecting a Highest Growth Rate During the Forecast Period?
Which end user segment holds the largest share of the Global Vaccines Market?