Contract Research Organization Services Market / CRO Services Market by Type (Early Phase, Clinical, Lab, Consulting, Data Management), Therapeutic Area (Cancer, Infectious Disease, Neurology, Dermatology, Immunology, Hematology, Vaccines, CGT) - Global Forecast to 2029
The contract research organization (CRO) services market is projected to reach USD 129.8 billion by 2029 from an estimated USD 82.0 billion in 2024, at a CAGR of 9.6% during the forecast period.
Clinical research efforts have been progressively outsourced to CROs during the past few years. Factors including cost effectiveness, access to technologically advanced infrastructure and regulatory know-how provided by Contract research organisations help market players predict a continuous increase in outsourcing. Moreover, a rising therapeutic pipeline and a changing scene of drug research help to explain market expansion. For businesses in the Contract Research Organisation (CRO) Services industry, the more emphasis on research and development creates opportunities.
Contract Research Organization Services Market Size, Dynamics & Ecosystem

To know about the assumptions considered for the study, Request for Free Sample Report
Contract Research Organization (CRO) Services Market Dynamics
MARKET DRIVER: Increasing number of drugs in pipeline
For the past eight to ten years, the potency of the pipeline has been expanding continuously due to the desire for novel therapies for many indications. Drug candidates under clinical development grew in numbers on the pipeline. For instance, within ten years the research and development pipeline doubled from 10,479 pharmaceuticals in 2013 to 21,292 in 2023. As a result, during the past few years, the percentage of outsourcing of drug development activities has also been rising. With an eye towards using the skills of CROs—including necessary infrastructure, therapeutic expertise, and regulatory experience—the sponsors—including academic institutions and biotechnology and pharmaceutical companies—outsource drug development activities. Consequently, the expansion of the CRO services market results from the increase in the R&D pipeline.
OPPORTUNITY: Growing focus on precision/ personalized medicine
As the benefits offered by personalized medicines have been recognized, the research and development initiatives in the area of precision/ personalized medicine have been growing largely. There has been a consistent growth in the number of products approved as personalized medicines by the US FDA. For instance, the US FDA has approved more than 50 drugs as personalized medicines between 2020 and 2022 which was merely 11 drugs in 2019. Additionally, CROs have also started offering special services for precision/ personalized medicines as well. This is attracting pharmaceutical and Biotech compenies to outsource these trials from CROs as well. Some instances of companies offering associated services include Labcorp, IQVIA, among others.
CHALLENGE: Patient recruitment and retention
Patient recruitment is the process of enrolling individuals for various phases of a clinical research. It is rather important for the result of the clinical investigation. Any clinical study's effectiveness depends critically on efficient patient recruitment and retention all through the trial. From the whole medication development process, patient recruiting has always presented a difficulty. This is mostly the result of various factors, including ignorance, complexity in the inclusion/exclusion criteria, and some longer-lasting trials that provide difficulties for retention. Furthermore another element causing difficulties in patient recruitment is a lack of knowledge about the viewpoint of patients. Decentralised clinical trials (DCT), however, are supposed to reduce the influence of this problem. Since they give time and place liberty to the subjects of the clinical studies, decentralised trials help to effectively recruit patients. Therefore, using DCT more often could help to lessen the consequences of this difficulty in the next years.
Leading organisations in the industry have been running for several years with diversified service portfolios, modern technologies, and powerful worldwide sales and marketing systems. Among the well-known companies in the CRO Services market are IQVIA Inc. (US), Thermo Fisher Scientific Inc. (US), Iicon Plc (Ireland), WuXi AppTec (China), Syneos Health (US), Charles River Laboratories (US), Fortrea, Inc. (US), Pharmaron (China), Eurofins Scientific (Luxembourg) and Medpace (US).
Contract Research Organization Services Market Segmentation & Geographical Spread

To know about the assumptions considered for the study, download the pdf brochure
The clinical research services accounted for the largest share of the type segment in overall Contract Research Organization (CRO) Services in 2023.
On the basis of type, the CRO Services market is segmented into Clinical Research Services, Early Phase Development Services, Laboratory Services, Consulting Services and Data Management Services. In 2023, the clinical research services segment accounted for the largest share of the CRO Services market owing to a major factor including growing number of clinical trials from phase 1 to 4 and an increase in the outsourcing of clinical research and development activities among others.
The oncology subsegment of the therapeutic area segment dominated the overall CRO Services market in 2023.
Based on Therapeutic areas, the CRO Services market is segmented into Oncology, Infectious Diseases, CVS Disorders, Neurology, Vaccines, Metabolic Disorders/Endocrinology, Immunological Disorders, Psychiatric, Respiratory Disorders, Dermatology, Ophthalmology, Gastrointestinal diseases, Genitourinary and Women's health, Haematology, and other therapy areas. Owing to factors including the steadily rising number of clinical trials against oncology indications, growing research and development funding channeled for oncology drug development initiatives, and an increasing frequency of various types of cancers in the upcoming years, oncology accounted for the biggest market share.
North America was the largest market for the overall CRO Services market in 2023 and also during the forecast period.
Geographically, the CRO Services market is segmented into North America, Europe, Asia Pacific, Latin America, Middle East and Africa. The CRO Services market was dominated by North America in 2023, and this dominance is anticipated to continue throughout the forecast period between 2024 and 2029. The market for CRO Services is expanding in the region as a result of factors like growing size of the pharmaceutical industry in the region, and large funds dedicated to pharma R&D, among others.
Key Market Players
The prominent players in the CRO Services market are IQVIA Inc. (US), Laboratory Corporation of America Holdings (US), Thermo Fisher Scientific Inc. (US), ICON Plc (Ireland), WuXi AppTec (China), Syneos Health (US), Charles River Laboratories (US), Fortrea, Inc. (US), Pharmaron (China), Eurofins Scientific (Luxembourg) and Medpace (US) among others.
Scope of the Contract Research Organization Services Industry
|
Report Metric |
Details |
|
Market Revenue in 2024 |
$82.0 billion |
|
Projected Revenue by 2029 |
$129.8 billion |
|
Revenue Rate |
Poised to Grow at a CAGR of 9.6% |
|
Market Driver |
Increasing number of drugs in pipeline |
|
Market Opportunity |
Growing focus on precision/ personalized medicine |
This report categorizes the Contract Research Organization Services Market to forecast revenue and analyze trends in each of the following submarkets:
By Type
-
Clinical Research Services
-
By Phase
- Phase III
- Phase II
- Phase I
- Phase IV
-
By Study Design (For Phase III & IV)
- Interventional
- Real World Evidence (RWE)
-
By Phase
-
Early phase development Services
- Chemistry, Manufacturing and Controls Services
-
Preclinical Services
- Pharmacokinetics/ Pharmacodynamics Services
- Toxicology Testing Services
- Other Preclinical Services
- Discovery Studies
-
Laboratory Services
-
Analytical Testing Services
- Physical Characterization Services
- Raw Material Testing Services
- Batch Release Testing Services
- Stability Testing Services
- Other Analytical Testing Services
- Bioanalytical Testing Services
-
Analytical Testing Services
- Consulting Services
- Data Management Services
By Therapeutic Area
- Oncology
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Other Cancer
- Infectious Diseases
- CVS Disorders
- Neurology
- Vaccines
- Metabolic Disorders/Endocrinology
- Immunological Disorders
- Psychiatry
- Respiratory Disorders
- Dermatology
- Ophthalmology
- Gastrointestinal Diseases
- Genitourinary & Women’s Health
- Hematology
- Other Therapeutic Areas
By End User
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Academic Institutes
By Region
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe (RoE)
-
Asia Pacific (APAC)
- China
- India
- Japan
- Australia
- South Korea
- New Zealand
- Rest of Asia Pacific (RoAPAC)
-
Latin America
- Brazil
- Mexico
- Rest of Latin America
-
Middle East and Africa
- Middle East
- Africa
Recent Developments
- In October 2023, IQVIA Inc. (US) and argenx (US) entered a strategic collaboration to support treatment for patients suffering from rare autoimmune disorders through innovative and comprehensive technology-enabled pharmacovigilance (PV) safety services and solutions.
- In October 2023, LabCorp announced the acquisition of the outreach laboratory business and selected operating assets of Baystate Health, comprising laboratory service centres operated by Baystate Health throughout Massachusetts, US.
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the global contract research organization (CRO) services Market?
The global contract research organization (CRO) services market boasts a total revenue value of $129.8 Billion by 2029.
What is the estimated growth rate (CAGR) of the global Contract research organization (CRO) services Market?
The global contract research organization (CRO) services market has an estimated compound annual growth rate (CAGR) of 9.6% and a revenue size in the region of $82.0 Billion in 2024. .
To speak to our analyst for a discussion on the above findings, click Speak to Analyst


- 5.1 INTRODUCTION
-
5.2 MARKET DYNAMICSDRIVERS- Rising pharmaceutical R&D expenditure and increasing drug pipeline- Technological advancements in clinical trials and innovative trial designs- High cost of in-house drug developmentOPPORTUNITIES- Favorable growth prospects for biologics and biosimilars market- Need for novel clinical trial designs for complex cell & gene therapies- Emergence of hybrid models with CRO-CDMO partnerships- Growing focus on personalized/precision medicine- Development of next-generation biotherapeutic productsCHALLENGES- Lack of adequate patient recruitment and retention for clinical trials- Changing clinical trial complexities
-
5.3 MARKET TRENDSINDUSTRY CONSOLIDATIONDECENTRALIZED CLINICAL TRIALSREAL-WORLD DATA AND REAL-WORLD EVIDENCEINCREASING INVOLVEMENT OF ARTIFICIAL INTELLIGENCE
-
5.4 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES
- 5.5 PRICING ANALYSIS
- 5.6 VALUE CHAIN ANALYSIS
-
5.7 ECOSYSTEM ANALYSIS
- 5.8 TECHNOLOGY ANALYSIS
-
5.9 CASE STUDY ANALYSISPPD TO LEVERAGE ITS PRECLARUS TECHNOLOGY FOR GENERATING CLEANER DATA, FASTER CLINICAL TRIALS, AND LESSER SITE BURDENIQVIA TO OFFER AI-POWERED MODELING FOR BETTER PATIENT IDENTIFICATIONUSE OF LAB DATA INSIGHTS TO IMPROVE POPULATION MANAGEMENT OF CHRONIC KIDNEY DISEASESIQVIA TO LEVERAGE ARTIFICIAL INTELLIGENCE AND MACHINE LEARNING FOR IMPROVED SERVICE OFFERINGS
- 5.10 KEY CONFERENCES & EVENTS
-
5.11 REGULATORY ANALYSISREGULATORY ANALYSISREGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONSREGULATORY SCENARIO FOR DRUG APPROVALS AND CGMP PROCEDURES
-
5.12 PORTER’S FIVE FORCES ANALYSISTHREAT OF NEW ENTRANTSTHREAT OF SUBSTITUTESBARGAINING POWER OF BUYERSBARGAINING POWER OF SUPPLIERSINTENSITY OF COMPETITIVE RIVALRY
-
5.13 KEY STAKEHOLDERS & BUYING CRITERIAKEY STAKEHOLDERS IN BUYING PROCESSBUYING CRITERIA
- 6.1 INTRODUCTION
-
6.2 CLINICAL RESEARCH SERVICESCLINICAL TRIAL SERVICES MARKET, BY PHASEPHASE III CLINICAL RESEARCH SERVICES- Increased use of clinical resources and advanced technologies to drive segmentPHASE II CLINICAL RESEARCH SERVICES- Rising number of pipeline products to propel marketPHASE I CLINICAL RESEARCH SERVICES- Growing R&D pipeline of pharmaceutical companies to support segmentPHASE IV CLINICAL RESEARCH SERVICES- Increasing number of CROs providing post-marketing surveillance to drive segmentCLINICAL RESEARCH SERVICES MARKET, BY STUDY DESIGN- Interventional studies- Real world evidence studies
-
6.3 EARLY-PHASE DEVELOPMENT SERVICESCHEMISTRY, MANUFACTURING, AND CONTROL SERVICES- Growing need to meet regulatory norms in drug development to drive segmentPRECLINICAL SERVICES- Pharmacokinetics/Pharmacodynamics- Toxicology testing- Other preclinical servicesDISCOVERY STUDIES- Increasing reliance on CRO services for target identification and validation to propel segment
-
6.4 LABORATORY SERVICESANALYTICAL TESTING- Physical characterization- Raw material testing- Batch-release testing- Stability testing- Other analytical testingBIOANALYTICAL TESTING- Rising significance of accurate PK/PD studies to drive segment
-
6.5 CONSULTING SERVICESGROWING ADOPTION OF CONSULTING SERVICES FOR FASTER REGULATORY APPROVALS TO SUPPORT MARKET
-
6.6 DATA MANAGEMENT SERVICESDATA MANAGEMENT SERVICES TO COMMAND HIGHEST GROWTH RATE DURING FORECAST PERIOD
- 7.1 INTRODUCTION
-
7.2 ONCOLOGYBREAST CANCER- Strong R&D pipeline for breast cancer drugs to drive segmentLUNG CANCER- Increasing focus on research for advanced treatment to drive segmentPROSTATE CANCER- Rising number of research initiatives to propel marketCOLORECTAL CANCER- Increasing drug research activities to support marketOTHER CANCERS
-
7.3 INFECTIOUS DISEASESGROWING INCIDENCE OF CHRONIC INFECTIONS TO SUPPORT MARKET
-
7.4 CARDIOVASCULAR SYSTEM DISORDERSINCREASING PREVALENCE OF CARDIOVASCULAR DISEASES TO DRIVE MARKET
-
7.5 NEUROLOGYINCREASING INVESTMENTS IN R&D TO DRIVE MARKET
-
7.6 METABOLIC DISORDERS/ENDOCRINOLOGYINCREASING NUMBER OF DIABETES PATIENTS TO BOOST MARKET
-
7.7 IMMUNOLOGICAL DISORDERSGROWING CLINICAL RESEARCH AND RISING NUMBER OF DRUGS IN R&D PIPELINE TO DRIVE MARKET
-
7.8 VACCINESGROWING PREVALENCE OF INFECTIOUS DISEASES AND RISING GOVERNMENT FUNDING TO SUPPORT MARKET
-
7.9 PSYCHIATRYGROWING CASES OF PSYCHIATRIC DISORDERS AND INCREASING INVESTMENT IN R&D TO DRIVE MARKET
-
7.10 RESPIRATORY DISORDERSRISING INCIDENCE OF UNDIAGNOSED RESPIRATORY DISORDERS AMONG CHILDREN TO PROPEL MARKET
-
7.11 DERMATOLOGYGROWING FOCUS ON DRUG DEVELOPMENT AGAINST VARIOUS SKIN CONDITIONS TO SUPPORT MARKET
-
7.12 OPHTHALMOLOGYRISING NUMBER OF PIPELINE DRUGS AND GROWING FOCUS ON R&D TO DRIVE MARKET
-
7.13 GASTROINTESTINAL DISEASESINCREASING LEVELS OF OBESITY WITH LIFESTYLE AND DIETARY CHANGES TO DRIVE MARKET
-
7.14 GENITOURINARY & WOMEN’S HEALTHRISING PREVALENCE OF CHRONIC DISORDERS AND INCREASING NUMBER OF PIPELINE DRUGS TO PROPEL MARKET
-
7.15 HEMATOLOGYGROWING PREVALENCE OF BLOOD-RELATED DISEASES TO SUPPORT MARKET
- 7.16 OTHER THERAPEUTIC AREAS
-
7.17 CUSTOMIZATIONCELL & GENE THERAPY- Growing investments for advanced research initiatives to support marketRARE DISEASES- Rising number of R&D activities for rare disease treatment to drive marketBIOSIMILARS- Growing focus on biosimilars and patent expiration of biologics to drive market- Monoclonal antibodies- Insulin- CSF- Erythropoietin- Other biosimilars
- 8.1 INTRODUCTION
-
8.2 PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIESSTABLE R&D PIPELINES AND INCREASED INVESTMENTS TO DRIVE MARKET
-
8.3 MEDICAL DEVICE COMPANIESINCREASING NUMBER OF GROWTH INITIATIVES BY MEDTECH ORGANIZATIONS TO DRIVE MARKET
-
8.4 ACADEMIC INSTITUTESRISING NUMBER OF COLLABORATIONS BETWEEN CONTRACT RESEARCH ORGANIZATIONS AND ACADEMIA TO DRIVE MARKET
- 9.1 INTRODUCTION
-
9.2 NORTH AMERICANORTH AMERICA: RECESSION IMPACTUS- US to dominate North American CRO services market during forecast periodCANADA- Short approval time and favorable government R&D investments to drive market
-
9.3 EUROPEEUROPE: RECESSION IMPACTGERMANY- Growing R&D spending and favorable government policies to propel marketUK- Growth in R&D expenditure in pharmaceutical industry to boost marketFRANCE- Government support for effective drug research to drive market growthITALY- Increased availability of funding for drug discovery and development to drive marketSPAIN- Growing R&D expenditure to boost marketREST OF EUROPE
-
9.4 ASIA PACIFICASIA PACIFIC: RECESSION IMPACTCHINA- Low manufacturing cost and increased establishment of R&D centers to drive marketINDIA- Growth in pharmaceutical industry and favorable scenario for foreign direct investment to propel marketJAPAN- Decline in number of clinical trials to limit marketAUSTRALIA- Favorable tax incentives and cash rebates to pharmaceutical companies to support marketSOUTH KOREA- Favorable government initiatives for drug development to drive marketNEW ZEALAND- Rising number of key players and growing R&D activities to support marketREST OF ASIA PACIFIC
-
9.5 LATIN AMERICALATIN AMERICA: RECESSION IMPACTBRAZIL- Increased government investments in pharmaceutical R&D to drive marketMEXICO- Favorable government policies for R&D and manufacturing to drive marketREST OF LATIN AMERICA
-
9.6 MIDDLE EAST & AFRICAMIDDLE EAST & AFRICA: RECESSION IMPACTMIDDLE EAST- Increase in clinical research activities to drive marketAFRICA- Growth in pharmaceutical industry to support market

- 10.1 INTRODUCTION
- 10.2 KEY STRATEGIES/RIGHT TO WIN
- 10.3 REVENUE SHARE ANALYSIS
- 10.4 MARKET SHARE ANALYSIS
-
10.5 COMPANY EVALUATION MATRIXSTARSEMERGING LEADERSPERVASIVE PLAYERSPARTICIPANTSCOMPANY FOOTPRINT
-
10.6 START-UP/SME EVALUATION MATRIXPROGRESSIVE COMPANIESRESPONSIVE COMPANIESDYNAMIC COMPANIESSTARTING BLOCKSCOMPETITIVE BENCHMARKING
-
10.7 COMPETITIVE SCENARIOS & TRENDSKEY SERVICES LAUNCHESKEY DEALSOTHER KEY DEVELOPMENTS
-
11.1 KEY PLAYERSIQVIA INC.- Business overview- Services/Solutions offered- Recent developments- MnM viewICON PLC- Business overview- Services/Solutions offered- Recent developments- MnM viewTHERMO FISHER SCIENTIFIC INC.- Business overview- Services/Solutions offered- Recent developments- MnM viewLABORATORY CORPORATION OF AMERICA HOLDINGS- Business overview- Services/Solutions offered- Recent developments- MnM viewSYNEOS HEALTH- Business overview- Services/Solutions offered- Recent developments- MnM viewWUXI APPTEC- Business overview- Services/Solutions offered- Recent developments- MnM viewCHARLES RIVER LABORATORIES- Business overview- Services/Solutions offered- Recent developments- MnM viewPAREXEL INTERNATIONAL CORPORATION- Business overview- Services/Solutions offered- Recent developmentsPHARMARON- Business overview- Services/Solutions offered- Recent developmentsFORTREA, INC.- Business overview- Services/Solutions offered- Recent developmentsMEDPACE- Business overview- Services/Solutions offeredSGS SA- Business overview- Services/Solutions offered- Recent developmentsFRONTAGE LABS- Business overview- Services/Solutions offered- Recent developmentsEUROFINS SCIENTIFIC- Business overview- Services/Solutions offered- Recent developmentsPSI CRO AG- Business overview- Services/Solutions offered- Recent developmentsBIOAGILE- Business overview- Services/Solutions offeredFIRMA CLINICAL RESEARCH- Business overview- Services/Solutions offeredACCULAB LIFE SCIENCES- Business overview- Services/Solutions offeredNOVOTECH- Business overview- Services/Solutions offered- Recent developmentsKCR S.A.- Business overview- Services/Solutions offered- Recent developmentsLINICAL- Business overview- Services/Solutions offered- Recent developmentsADVANCED CLINICAL- Business overview- Services/Solutions offered- Recent developments
-
11.2 OTHER PLAYERSALLUCENTCLINICAL TRIAL SERVICEGUIRES INC. (PEPGRA HEALTHCARE PVT. LTD.)WORLDWIDE CLINICAL TRIALSCTI CLINICAL TRIAL & CONSULTING
- 12.1 DISCUSSION GUIDE
- 12.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
- 12.3 CUSTOMIZATION OPTIONS
- 12.4 RELATED REPORTS
- 12.5 AUTHOR DETAILS
- TABLE 1 GLOBAL INFLATION RATE PROJECTION, 2021–2028 (% GROWTH)
- TABLE 2 US HEALTH EXPENDITURE, 2019–2022 (USD MILLION)
- TABLE 3 US HEALTH EXPENDITURE, 2023–2030 (USD MILLION)
- TABLE 4 IMPACT ANALYSIS: CRO SERVICES MARKET
- TABLE 5 LIST OF MERGERS AND ACQUISITIONS
- TABLE 6 PRICING ILLUSTRATION FOR FIRST IN-HUMAN TRIAL IN HEALTHY VOLUNTEERS IN EUROPE
- TABLE 7 ROLE IN ECOSYSTEM: CRO SERVICES MARKET
- TABLE 8 DETAILED LIST OF KEY CONFERENCES & EVENTS, 2024
- TABLE 9 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 10 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 11 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 12 REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 13 PORTER’S FIVE FORCES ANALYSIS: CRO SERVICES MARKET
- TABLE 14 CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 15 NUMBER OF REGISTERED AND RECRUITING CLINICAL STUDIES, BY LOCATION (AS OF DECEMBER 21, 2023)
- TABLE 16 EXAMPLES OF CLINICAL RESEARCH SERVICES OFFERED BY MARKET PLAYERS
- TABLE 17 CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 18 CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 19 NORTH AMERICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 20 EUROPE: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 21 ASIA PACIFIC: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 22 LATIN AMERICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 23 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 24 EXAMPLES OF DRUGS IN PHASE III OF CLINICAL TRIALS, 2023
- TABLE 25 PHASE III CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 26 NORTH AMERICA: PHASE III CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 27 EUROPE: PHASE III CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 28 ASIA PACIFIC: PHASE III CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 29 LATIN AMERICA: PHASE III CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 30 MIDDLE EAST & AFRICA: PHASE III CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 31 EXAMPLES OF DRUGS IN PHASE II OF CLINICAL TRIALS, 2023
- TABLE 32 PHASE II CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 33 NORTH AMERICA: PHASE II CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 34 EUROPE: PHASE II CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 35 ASIA PACIFIC: PHASE II CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 36 LATIN AMERICA: PHASE II CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 37 MIDDLE EAST & AFRICA: PHASE II CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 38 EXAMPLES OF DRUGS IN PHASE I OF CLINICAL TRIALS, 2023
- TABLE 39 PHASE I CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 40 NORTH AMERICA: PHASE I CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 41 EUROPE: PHASE I CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 42 ASIA PACIFIC: PHASE I CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 43 LATIN AMERICA: PHASE I CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 44 MIDDLE EAST & AFRICA: PHASE I CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 45 EXAMPLES OF DRUGS IN PHASE IV OF CLINICAL TRIALS, 2023
- TABLE 46 PHASE IV CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 47 NORTH AMERICA: PHASE IV CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 48 EUROPE: PHASE IV CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 49 ASIA PACIFIC: PHASE IV CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 50 LATIN AMERICA: PHASE IV CLINICAL RESEARCH SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 51 MIDDLE EAST & AFRICA: PHASE IV CLINICAL RESEARCH SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 52 CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY STUDY DESIGN, 2022–2029 (USD MILLION)
- TABLE 53 CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 54 INTERVENTIONAL STUDIES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 55 REAL WORLD EVIDENCE STUDIES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 56 EXAMPLES OF EARLY-PHASE DEVELOPMENT SERVICES OFFERED BY MARKET PLAYERS
- TABLE 57 CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 58 CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 59 NORTH AMERICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 60 EUROPE: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 61 ASIA PACIFIC: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 62 LATIN AMERICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 63 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 64 CHEMISTRY, MANUFACTURING, AND CONTROL SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 65 NORTH AMERICA: CHEMISTRY, MANUFACTURING, AND CONTROL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 66 EUROPE: CHEMISTRY, MANUFACTURING, AND CONTROL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 67 ASIA PACIFIC: CHEMISTRY, MANUFACTURING, AND CONTROL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 68 LATIN AMERICA: CHEMISTRY, MANUFACTURING, AND CONTROL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 69 MIDDLE EAST & AFRICA: CHEMISTRY, MANUFACTURING, AND CONTROL SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 70 PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 71 PRECLINICAL SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 72 NORTH AMERICA: PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 73 EUROPE: PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 74 ASIA PACIFIC: PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 75 LATIN AMERICA: PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 76 MIDDLE EAST & AFRICA: PRECLINICAL SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 77 PHARMACOKINETICS/PHARMACODYNAMICS MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 78 NORTH AMERICA: PHARMACOKINETICS/PHARMACODYNAMICS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 79 EUROPE: PHARMACOKINETICS/PHARMACODYNAMICS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 80 ASIA PACIFIC: PHARMACOKINETICS/PHARMACODYNAMICS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 81 LATIN AMERICA: PHARMACOKINETICS/PHARMACODYNAMICS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 82 MIDDLE EAST & AFRICA: PHARMACOKINETICS/PHARMACODYNAMICS MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 83 TOXICOLOGY TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 84 NORTH AMERICA: TOXICOLOGY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 85 EUROPE: TOXICOLOGY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 86 ASIA PACIFIC: TOXICOLOGY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 87 LATIN AMERICA: TOXICOLOGY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 88 MIDDLE EAST & AFRICA: TOXICOLOGY TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 89 OTHER PRECLINICAL SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 90 NORTH AMERICA: OTHER PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 91 EUROPE: OTHER PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 92 ASIA PACIFIC: OTHER PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 93 LATIN AMERICA: OTHER PRECLINICAL SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 94 MIDDLE EAST & AFRICA: OTHER PRECLINICAL SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 95 DISCOVERY STUDIES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 96 NORTH AMERICA: DISCOVERY STUDIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 97 EUROPE: DISCOVERY STUDIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 98 ASIA PACIFIC: DISCOVERY STUDIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 99 LATIN AMERICA: DISCOVERY STUDIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 100 MIDDLE EAST & AFRICA: DISCOVERY STUDIES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 101 EXAMPLES OF LABORATORY SERVICES OFFERED BY MARKET PLAYERS
- TABLE 102 CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 103 CRO SERVICES MARKET FOR LABORATORY SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 104 NORTH AMERICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 105 EUROPE: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 106 ASIA PACIFIC: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 107 LATIN AMERICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 108 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 109 ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 110 ANALYTICAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 111 NORTH AMERICA: ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 112 EUROPE: ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 113 ASIA PACIFIC: ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 114 LATIN AMERICA: ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 115 MIDDLE EAST & AFRICA: ANALYTICAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 116 PHYSICAL CHARACTERIZATION MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 117 NORTH AMERICA: PHYSICAL CHARACTERIZATION MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 118 EUROPE: PHYSICAL CHARACTERIZATION MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 119 ASIA PACIFIC: PHYSICAL CHARACTERIZATION MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 120 LATIN AMERICA: PHYSICAL CHARACTERIZATION MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 121 MIDDLE EAST & AFRICA: PHYSICAL CHARACTERIZATION MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 122 RAW MATERIAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 123 NORTH AMERICA: RAW MATERIAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 124 EUROPE: RAW MATERIAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 125 ASIA PACIFIC: RAW MATERIAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 126 LATIN AMERICA: RAW MATERIAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 127 MIDDLE EAST & AFRICA: RAW MATERIAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 128 BATCH-RELEASE TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 129 NORTH AMERICA: BATCH-RELEASE TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 130 EUROPE: BATCH-RELEASE TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 131 ASIA PACIFIC: BATCH-RELEASE TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 132 LATIN AMERICA: BATCH-RELEASE TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 133 MIDDLE EAST & AFRICA: BATCH-RELEASE TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 134 STABILITY TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 135 NORTH AMERICA: STABILITY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 136 EUROPE: STABILITY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 137 ASIA PACIFIC: STABILITY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 138 LATIN AMERICA: STABILITY TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 139 MIDDLE EAST & AFRICA: STABILITY TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 140 OTHER ANALYTICAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 141 NORTH AMERICA: OTHER ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 142 EUROPE: OTHER ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 143 ASIA PACIFIC: OTHER ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 144 LATIN AMERICA: OTHER ANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 145 MIDDLE EAST & AFRICA: OTHER ANALYTICAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 146 BIOANALYTICAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 147 NORTH AMERICA: BIOANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 148 EUROPE: BIOANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 149 ASIA PACIFIC: BIOANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 150 LATIN AMERICA: BIOANALYTICAL TESTING MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 151 MIDDLE EAST & AFRICA: BIOANALYTICAL TESTING MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 152 EXAMPLES OF CONSULTING SERVICES OFFERED BY MARKET PLAYERS
- TABLE 153 CRO SERVICES MARKET FOR CONSULTING SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 154 NORTH AMERICA: CRO SERVICES MARKET FOR CONSULTING SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 155 EUROPE: CRO SERVICES MARKET FOR CONSULTING SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 156 ASIA PACIFIC: CRO SERVICES MARKET FOR CONSULTING SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 157 LATIN AMERICA: CRO SERVICES MARKET FOR CONSULTING SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 158 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR CONSULTING SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 159 EXAMPLES OF DATA MANAGEMENT SERVICES OFFERED BY MARKET PLAYERS
- TABLE 160 CRO SERVICES MARKET FOR DATA MANAGEMENT SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 161 NORTH AMERICA: CRO SERVICES MARKET FOR DATA MANAGEMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 162 EUROPE: CRO SERVICES MARKET FOR DATA MANAGEMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 163 ASIA PACIFIC: CRO SERVICES MARKET FOR DATA MANAGEMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 164 LATIN AMERICA: CRO SERVICES MARKET FOR DATA MANAGEMENT SERVICES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 165 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR DATA MANAGEMENT SERVICES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 166 CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 167 NUMBER OF NEW CANCER CASES, BY TYPE, 2020 VS. 2040
- TABLE 168 LIST OF FDA DRUGS APPROVED FOR ONCOLOGY, 2023
- TABLE 169 CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 170 CRO SERVICES MARKET FOR ONCOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 171 NORTH AMERICA: CRO SERVICES MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 172 EUROPE: CRO SERVICES MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 173 ASIA PACIFIC: CRO SERVICES MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 174 LATIN AMERICA: CRO SERVICES MARKET FOR ONCOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 175 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR ONCOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 176 BREAST CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 177 NORTH AMERICA: BREAST CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 178 EUROPE: BREAST CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 179 ASIA PACIFIC: BREAST CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 180 LATIN AMERICA: BREAST CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 181 MIDDLE EAST & AFRICA: BREAST CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 182 LUNG CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 183 NORTH AMERICA: LUNG CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 184 EUROPE: LUNG CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 185 ASIA PACIFIC: LUNG CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 186 LATIN AMERICA: LUNG CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 187 MIDDLE EAST & AFRICA: LUNG CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 188 PROSTATE CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 189 NORTH AMERICA: PROSTATE CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 190 EUROPE: PROSTATE CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 191 ASIA PACIFIC: PROSTATE CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 192 LATIN AMERICA: PROSTATE CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 193 MIDDLE EAST & AFRICA: PROSTRATE CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 194 COLORECTAL CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 195 NORTH AMERICA: COLORECTAL CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 196 EUROPE: COLORECTAL CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 197 ASIA PACIFIC: COLORECTAL CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 198 LATIN AMERICA: COLORECTAL CANCER MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 199 MIDDLE EAST & AFRICA: COLORECTAL CANCER MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 200 NUMBER OF DRUGS IN R&D PIPELINE FOR OTHER CANCERS, 2022 AND 2023
- TABLE 201 OTHER CANCERS MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 202 NORTH AMERICA: OTHER CANCERS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 203 EUROPE: OTHER CANCERS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 204 ASIA PACIFIC: OTHER CANCERS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 205 LATIN AMERICA: OTHER CANCERS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 206 MIDDLE EAST & AFRICA: OTHER CANCERS MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 207 CRO SERVICES MARKET FOR INFECTIOUS DISEASES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 208 NORTH AMERICA: CRO SERVICES MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 209 EUROPE: CRO SERVICES MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 210 ASIA PACIFIC: CRO SERVICES MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 211 LATIN AMERICA: CRO SERVICES MARKET FOR INFECTIOUS DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 212 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR INFECTIOUS DISEASES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 213 EXAMPLES OF PIPELINE DRUGS FOR CARDIOVASCULAR DISEASES (AS OF DECEMBER 2023)
- TABLE 214 CRO SERVICES MARKET FOR CARDIOVASCULAR SYSTEM DISORDERS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 215 NORTH AMERICA: CRO SERVICES MARKET FOR CARDIOVASCULAR SYSTEM DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 216 EUROPE: CRO SERVICES MARKET FOR CARDIOVASCULAR SYSTEM DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 217 ASIA PACIFIC: CRO SERVICES MARKET FOR CARDIOVASCULAR SYSTEM DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 218 LATIN AMERICA: CRO SERVICES MARKET FOR CARDIOVASCULAR SYSTEM DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 219 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR CARDIOVASCULAR SYSTEM DISORDERS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 220 CRO SERVICES MARKET FOR NEUROLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 221 NORTH AMERICA: CRO SERVICES MARKET FOR NEUROLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 222 EUROPE: CRO SERVICES MARKET FOR NEUROLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 223 ASIA PACIFIC: CRO SERVICES MARKET FOR NEUROLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 224 LATIN AMERICA: CRO SERVICES MARKET FOR NEUROLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 225 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR NEUROLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 226 CRO SERVICES MARKET FOR METABOLIC DISORDERS/ENDOCRINOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 227 NORTH AMERICA: CRO SERVICES MARKET FOR METABOLIC DISORDERS/ENDOCRINOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 228 EUROPE: CRO SERVICES MARKET FOR METABOLIC DISORDERS/ENDOCRINOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 229 ASIA PACIFIC: CRO SERVICES MARKET FOR METABOLIC DISORDERS/ENDOCRINOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 230 LATIN AMERICA: CRO SERVICES MARKET FOR METABOLIC DISORDERS/ENDOCRINOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 231 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR METABOLIC DISORDERS/ENDOCRINOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 232 EXAMPLES OF PIPELINE DRUGS FOR IMMUNOLOGICAL DISORDERS, 2023
- TABLE 233 CRO SERVICES MARKET FOR IMMUNOLOGICAL DISORDERS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 234 NORTH AMERICA: CRO SERVICES MARKET FOR IMMUNOLOGICAL DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 235 EUROPE: CRO SERVICES MARKET FOR IMMUNOLOGICAL DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 236 ASIA PACIFIC: CRO SERVICES MARKET FOR IMMUNOLOGICAL DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 237 LATIN AMERICA: CRO SERVICES MARKET FOR IMMUNOLOGICAL DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 238 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR IMMUNOLOGICAL DISORDERS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 239 CRO SERVICES MARKET FOR VACCINES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 240 NORTH AMERICA: CRO SERVICES MARKET FOR VACCINES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 241 EUROPE: CRO SERVICES MARKET FOR VACCINES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 242 ASIA PACIFIC: CRO SERVICES MARKET FOR VACCINES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 243 LATIN AMERICA: CRO SERVICES MARKET FOR VACCINES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 244 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR VACCINES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 245 CRO SERVICES MARKET FOR PSYCHIATRY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 246 NORTH AMERICA: CRO SERVICES MARKET FOR PSYCHIATRY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 247 EUROPE: CRO SERVICES MARKET FOR PSYCHIATRY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 248 ASIA PACIFIC: CRO SERVICES MARKET FOR PSYCHIATRY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 249 LATIN AMERICA: CRO SERVICES MARKET FOR PSYCHIATRY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 250 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR PSYCHIATRY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 251 LIST OF PIPELINE DRUGS FOR RESPIRATORY DISEASES, 2023
- TABLE 252 CRO SERVICES MARKET FOR RESPIRATORY DISORDERS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 253 NORTH AMERICA: CRO SERVICES MARKET FOR RESPIRATORY DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 254 EUROPE: CRO SERVICES MARKET FOR RESPIRATORY DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 255 ASIA PACIFIC: CRO SERVICES MARKET FOR RESPIRATORY DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 256 LATIN AMERICA: CRO SERVICES MARKET FOR RESPIRATORY DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 257 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR RESPIRATORY DISORDERS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 258 LIST OF PIPELINE DRUGS FOR SKIN DISEASES, 2023
- TABLE 259 CRO SERVICES MARKET FOR DERMATOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 260 NORTH AMERICA: CRO SERVICES MARKET FOR DERMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 261 EUROPE: CRO SERVICES MARKET FOR DERMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 262 ASIA PACIFIC: CRO SERVICES MARKET FOR DERMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 263 LATIN AMERICA: CRO SERVICES MARKET FOR DERMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 264 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR DERMATOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 265 CRO SERVICES MARKET FOR OPHTHALMOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 266 NORTH AMERICA: CRO SERVICES MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 267 EUROPE: CRO SERVICES MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 268 ASIA PACIFIC: CRO SERVICES MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 269 LATIN AMERICA: CRO SERVICES MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 270 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR OPHTHALMOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 271 CRO SERVICES MARKET FOR GASTROINTESTINAL DISEASES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 272 NORTH AMERICA: CRO SERVICES MARKET FOR GASTROINTESTINAL DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 273 EUROPE: CRO SERVICES MARKET FOR GASTROINTESTINAL DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 274 ASIA PACIFIC: CRO SERVICES MARKET FOR GASTROINTESTINAL DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 275 LATIN AMERICA: CRO SERVICES MARKET FOR GASTROINTESTINAL DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 276 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR GASTROINTESTINAL DISEASES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 277 CRO SERVICES MARKET FOR GENITOURINARY & WOMEN’S HEALTH, BY REGION, 2022–2029 (USD MILLION)
- TABLE 278 NORTH AMERICA: CRO SERVICES MARKET FOR GENITOURINARY & WOMEN’S HEALTH, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 279 EUROPE: CRO SERVICES MARKET FOR GENITOURINARY & WOMEN’S HEALTH, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 280 ASIA PACIFIC: CRO SERVICES MARKET FOR GENITOURINARY & WOMEN’S HEALTH, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 281 LATIN AMERICA: CRO SERVICES MARKET FOR GENITOURINARY & WOMEN’S HEALTH, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 282 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR GENITOURINARY & WOMEN’S HEALTH, BY REGION, 2022–2029 (USD MILLION)
- TABLE 283 CRO SERVICES MARKET FOR HEMATOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 284 NORTH AMERICA: CRO SERVICES MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 285 EUROPE: CRO SERVICES MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 286 ASIA PACIFIC: CRO SERVICES MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 287 LATIN AMERICA: CRO SERVICES MARKET FOR HEMATOLOGY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 288 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR HEMATOLOGY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 289 CRO SERVICES MARKET FOR OTHER THERAPEUTIC AREAS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 290 NORTH AMERICA: CRO SERVICES MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 291 EUROPE: CRO SERVICES MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 292 ASIA PACIFIC: CRO SERVICES MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 293 LATIN AMERICA: CRO SERVICES MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 294 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR OTHER THERAPEUTIC AREAS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 295 CRO SERVICES MARKET FOR CELL & GENE THERAPY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 296 NORTH AMERICA: CRO SERVICES MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 297 EUROPE: CRO SERVICES MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 298 ASIA PACIFIC: CRO SERVICES MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 299 LATIN AMERICA: CRO SERVICES MARKET FOR CELL & GENE THERAPY, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 300 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR CELL & GENE THERAPY, BY REGION, 2022–2029 (USD MILLION)
- TABLE 301 CRO SERVICES MARKET FOR RARE DISEASES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 302 NORTH AMERICA: CRO SERVICES MARKET FOR RARE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 303 EUROPE: CRO SERVICES MARKET FOR RARE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 304 ASIA PACIFIC: CRO SERVICES MARKET FOR RARE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 305 LATIN AMERICA: CRO SERVICES MARKET FOR RARE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 306 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR RARE DISEASES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 307 CRO SERVICES MARKET FOR BIOSIMILARS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 308 NORTH AMERICA: CRO SERVICES MARKET FOR BIOSIMILARS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 309 EUROPE: CRO SERVICES MARKET FOR BIOSIMILARS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 310 ASIA PACIFIC: CRO SERVICES MARKET FOR BIOSIMILARS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 311 LATIN AMERICA: CRO SERVICES MARKET FOR BIOSIMILARS, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 312 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR BIOSIMILARS, BY REGION, 2022–2029 (USD MILLION)
- TABLE 313 CRO SERVICES MARKET FOR BIOSIMILARS, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 314 MONOCLONAL ANTIBODIES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 315 NORTH AMERICA: MONOCLONAL ANTIBODIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 316 EUROPE: MONOCLONAL ANTIBODIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 317 ASIA PACIFIC: MONOCLONAL ANTIBODIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 318 LATIN AMERICA: MONOCLONAL ANTIBODIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 319 MIDDLE EAST & AFRICA: MONOCLONAL ANTIBODIES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 320 INSULIN MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 321 NORTH AMERICA: INSULIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 322 EUROPE: INSULIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 323 ASIA PACIFIC: INSULIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 324 LATIN AMERICA: INSULIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 325 MIDDLE EAST & AFRICA: INSULIN MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 326 CSF MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 327 NORTH AMERICA: CSF MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 328 EUROPE: CSF MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 329 ASIA PACIFIC: CSF MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 330 LATIN AMERICA: CSF MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 331 MIDDLE EAST & AFRICA: CSF MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 332 ERYTHROPOIETIN MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 333 NORTH AMERICA: ERYTHROPOIETIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 334 EUROPE: ERYTHROPOIETIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 335 ASIA PACIFIC: ERYTHROPOIETIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 336 LATIN AMERICA: ERYTHROPOIETIN MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 337 MIDDLE EAST & AFRICA: ERYTHROPOIETIN MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 338 OTHER BIOSIMILARS MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 339 NORTH AMERICA: OTHER BIOSIMILARS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 340 EUROPE: OTHER BIOSIMILARS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 341 ASIA PACIFIC: OTHER BIOSIMILARS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 342 LATIN AMERICA: OTHER BIOSIMILARS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 343 MIDDLE EAST & AFRICA: OTHER BIOSIMILARS MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 344 CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 345 CRO SERVICES MARKET FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 346 NORTH AMERICA: CRO SERVICES MARKET FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 347 EUROPE: CRO SERVICES MARKET FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 348 ASIA PACIFIC: CRO SERVICES MARKET FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 349 LATIN AMERICA: CRO SERVICES MARKET FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 350 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 351 CRO SERVICES MARKET FOR MEDICAL DEVICE COMPANIES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 352 NORTH AMERICA: CRO SERVICES MARKET FOR MEDICAL DEVICE COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 353 EUROPE: CRO SERVICES MARKET FOR MEDICAL DEVICE COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 354 ASIA PACIFIC: CRO SERVICES MARKET FOR MEDICAL DEVICE COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 355 LATIN AMERICA: CRO SERVICES MARKET FOR MEDICAL DEVICE COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 356 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR MEDICAL DEVICE COMPANIES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 357 CRO SERVICES MARKET FOR ACADEMIC INSTITUTES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 358 NORTH AMERICA: CRO SERVICES MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 359 EUROPE: CRO SERVICES MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 360 ASIA PACIFIC: CRO SERVICES MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 361 LATIN AMERICA: CRO SERVICES MARKET FOR ACADEMIC INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 362 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR ACADEMIC INSTITUTES, BY REGION, 2022–2029 (USD MILLION)
- TABLE 363 CRO SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 364 NORTH AMERICA: CRO SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 365 NORTH AMERICA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 366 NORTH AMERICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 367 NORTH AMERICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 368 NORTH AMERICA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 369 NORTH AMERICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 370 NORTH AMERICA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 371 NORTH AMERICA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 372 NORTH AMERICA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 373 NORTH AMERICA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 374 US: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 375 US: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 376 US: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 377 US: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 378 US: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 379 US: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 380 US: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 381 US: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 382 US: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 383 CANADA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 384 CANADA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 385 CANADA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 386 CANADA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 387 CANADA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 388 CANADA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 389 CANADA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 390 CANADA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 391 CANADA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 392 EUROPE: CRO SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 393 EUROPE: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 394 EUROPE: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 395 EUROPE: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 396 EUROPE: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 397 EUROPE: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 398 EUROPE: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 399 EUROPE: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 400 EUROPE: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 401 EUROPE: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 402 GERMANY: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 403 GERMANY: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 404 GERMANY: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 405 GERMANY: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 406 GERMANY: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 407 GERMANY: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 408 GERMANY: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 409 GERMANY: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 410 GERMANY: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 411 UK: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 412 UK: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 413 UK: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 414 UK: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 415 UK: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 416 UK: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 417 UK: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 418 UK: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 419 UK: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 420 FRANCE: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 421 FRANCE: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 422 FRANCE: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 423 FRANCE: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 424 FRANCE: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 425 FRANCE: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 426 FRANCE: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 427 FRANCE: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 428 FRANCE: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 429 ITALY: NUMBER OF CLINICAL TRIALS IN ITALY, BY COMPANY (JANUARY 2022 VS. JANUARY 2023)
- TABLE 430 ITALY: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 431 ITALY: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 432 ITALY: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 433 ITALY: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 434 ITALY: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 435 ITALY: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 436 ITALY: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 437 ITALY: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 438 ITALY: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 439 SPAIN: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 440 SPAIN: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 441 SPAIN: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 442 SPAIN: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 443 SPAIN: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 444 SPAIN: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 445 SPAIN: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 446 SPAIN: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 447 SPAIN: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 448 REST OF EUROPE: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 449 REST OF EUROPE: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 450 REST OF EUROPE: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 451 REST OF EUROPE: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 452 REST OF EUROPE: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 453 REST OF EUROPE: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 454 REST OF EUROPE: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 455 REST OF EUROPE: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 456 REST OF EUROPE: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 457 ASIA PACIFIC: CRO SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 458 ASIA PACIFIC: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 459 ASIA PACIFIC: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 460 ASIA PACIFIC: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 461 ASIA PACIFIC: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 462 ASIA PACIFIC: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 463 ASIA PACIFIC: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 464 ASIA PACIFIC: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 465 ASIA PACIFIC: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 466 ASIA PACIFIC: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 467 CHINA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 468 CHINA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 469 CHINA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 470 CHINA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 471 CHINA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 472 CHINA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 473 CHINA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 474 CHINA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 475 CHINA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 476 INDIA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 477 INDIA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 478 INDIA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 479 INDIA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 480 INDIA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 481 INDIA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 482 INDIA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 483 INDIA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 484 INDIA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 485 JAPAN: TOTAL NUMBER OF CLINICAL TRIALS IN JAPAN, BY THERAPEUTIC AREA, 2020, 2022, AND 2023
- TABLE 486 JAPAN: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 487 JAPAN: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 488 JAPAN: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 489 JAPAN: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 490 JAPAN: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 491 JAPAN: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 492 JAPAN: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 493 JAPAN: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 494 JAPAN: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 495 AUSTRALIA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 496 AUSTRALIA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 497 AUSTRALIA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 498 AUSTRALIA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 499 AUSTRALIA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 500 AUSTRALIA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 501 AUSTRALIA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 502 AUSTRALIA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 503 AUSTRALIA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 504 SOUTH KOREA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 505 SOUTH KOREA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 506 SOUTH KOREA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 507 SOUTH KOREA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 508 SOUTH KOREA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 509 SOUTH KOREA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 510 SOUTH KOREA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 511 SOUTH KOREA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 512 SOUTH KOREA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 513 NEW ZEALAND: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 514 NEW ZEALAND: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 515 NEW ZEALAND: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 516 NEW ZEALAND: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 517 NEW ZEALAND: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 518 NEW ZEALAND: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 519 NEW ZEALAND: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 520 NEW ZEALAND: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 521 NEW ZEALAND: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 522 REST OF ASIA PACIFIC: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 523 REST OF ASIA PACIFIC: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 524 REST OF ASIA PACIFIC: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 525 REST OF ASIA PACIFIC: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 526 REST OF ASIA PACIFIC: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 527 REST OF ASIA PACIFIC: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 528 REST OF ASIA PACIFIC: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 529 REST OF ASIA PACIFIC: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 530 REST OF ASIA PACIFIC: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 531 LATIN AMERICA: CRO SERVICES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
- TABLE 532 LATIN AMERICA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 533 LATIN AMERICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 534 LATIN AMERICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 535 LATIN AMERICA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 536 LATIN AMERICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 537 LATIN AMERICA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 538 LATIN AMERICA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 539 LATIN AMERICA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 540 LATIN AMERICA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 541 BRAZIL: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 542 BRAZIL: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 543 BRAZIL: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 544 BRAZIL: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 545 BRAZIL: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 546 BRAZIL: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 547 BRAZIL: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 548 BRAZIL: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 549 BRAZIL: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 550 MEXICO: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 551 MEXICO: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 552 MEXICO: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 553 MEXICO: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 554 MEXICO: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 555 MEXICO: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 556 MEXICO: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 557 MEXICO: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 558 MEXICO: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 559 REST OF LATIN AMERICA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 560 REST OF LATIN AMERICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 561 REST OF LATIN AMERICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 562 REST OF LATIN AMERICA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 563 REST OF LATIN AMERICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 564 REST OF LATIN AMERICA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 565 REST OF LATIN AMERICA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 566 REST OF LATIN AMERICA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 567 REST OF LATIN AMERICA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 568 MIDDLE EAST & AFRICA: CRO SERVICES MARKET, BY REGION, 2022–2029 (USD MILLION)
- TABLE 569 MIDDLE EAST & AFRICA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 570 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 571 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 572 MIDDLE EAST & AFRICA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 573 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 574 MIDDLE EAST & AFRICA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 575 MIDDLE EAST & AFRICA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 576 MIDDLE EAST & AFRICA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 577 MIDDLE EAST & AFRICA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 578 MIDDLE EAST: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 579 MIDDLE EAST: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 580 MIDDLE EAST: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 581 MIDDLE EAST: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 582 MIDDLE EAST: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 583 MIDDLE EAST: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 584 MIDDLE EAST: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 585 MIDDLE EAST: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 586 MIDDLE EAST: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 587 AFRICA: CRO SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 588 AFRICA: CRO SERVICES MARKET FOR CLINICAL RESEARCH SERVICES, BY PHASE, 2022–2029 (USD MILLION)
- TABLE 589 AFRICA: CRO SERVICES MARKET FOR EARLY-PHASE DEVELOPMENT SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 590 AFRICA: PRECLINICAL SERVICES MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 591 AFRICA: CRO SERVICES MARKET FOR LABORATORY SERVICES, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 592 AFRICA: ANALYTICAL TESTING MARKET, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 593 AFRICA: CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2022–2029 (USD MILLION)
- TABLE 594 AFRICA: CRO SERVICES MARKET FOR ONCOLOGY, BY TYPE, 2022–2029 (USD MILLION)
- TABLE 595 AFRICA: CRO SERVICES MARKET, BY END USER, 2022–2029 (USD MILLION)
- TABLE 596 DEGREE OF COMPETITION: CRO SERVICES MARKET
- TABLE 597 INDUSTRY FOOTPRINT
- TABLE 598 SERVICE FOOTPRINT
- TABLE 599 REGIONAL FOOTPRINT
- TABLE 600 DETAILED LIST OF KEY START-UPS/SMES
- TABLE 601 COMPETITIVE BENCHMARKING OF START-UPS/SMES
- TABLE 602 KEY SERVICE LAUNCHES, JANUARY 2020–SEPTEMBER 2023
- TABLE 603 KEY DEALS, JANUARY 2020– SEPTEMBER 2023
- TABLE 604 KEY EXPANSIONS, JANUARY 2020– SEPTEMBER 2023
- TABLE 605 IQVIA INC.: COMPANY OVERVIEW
- TABLE 606 ICON PLC: COMPANY OVERVIEW
- TABLE 607 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
- TABLE 608 LABORATORY CORPORATION OF AMERICA HOLDINGS: COMPANY OVERVIEW
- TABLE 609 SYNEOS HEALTH: COMPANY OVERVIEW
- TABLE 610 WUXI APPTEC: COMPANY OVERVIEW
- TABLE 611 CHARLES RIVER LABORATORIES: COMPANY OVERVIEW
- TABLE 612 PAREXEL INTERNATIONAL CORPORATION: COMPANY OVERVIEW
- TABLE 613 PHARMARON: COMPANY OVERVIEW
- TABLE 614 FORTREA, INC.: COMPANY OVERVIEW
- TABLE 615 MEDPACE: COMPANY OVERVIEW
- TABLE 616 SGS SA: COMPANY OVERVIEW
- TABLE 617 FRONTAGE LABS: COMPANY OVERVIEW
- TABLE 618 EUROFINS SCIENTIFIC: COMPANY OVERVIEW
- TABLE 619 PSI CRO AG: COMPANY OVERVIEW
- TABLE 620 BIOAGILE: COMPANY OVERVIEW
- TABLE 621 FIRMA CLINICAL RESEARCH: COMPANY OVERVIEW
- TABLE 622 ACCULAB LIFE SCIENCES: COMPANY OVERVIEW
- TABLE 623 NOVOTECH: COMPANY OVERVIEW
- TABLE 624 KCR S.A.: COMPANY OVERVIEW
- TABLE 625 LINICAL: COMPANY OVERVIEW
- TABLE 626 ADVANCED CLINICAL: COMPANY OVERVIEW
- FIGURE 1 RESEARCH DESIGN
- FIGURE 2 SECONDARY DATA SOURCES
- FIGURE 3 BREAKDOWN OF PRIMARIES: SUPPLY- AND DEMAND-SIDE PARTICIPANTS
- FIGURE 4 MARKET SIZE ESTIMATION: SUPPLY-SIDE ANALYSIS, 2023
- FIGURE 5 MARKET SIZE ESTIMATION: APPROACH 1 (REVENUE SHARE ANALYSIS), 2023
- FIGURE 6 ILLUSTRATIVE EXAMPLE OF IQVIA INC.: REVENUE SHARE ANALYSIS, 2023
- FIGURE 7 MARKET VALIDATION FROM PRIMARY EXPERTS
- FIGURE 8 MARKET SIZE ESTIMATION: TOP-DOWN APPROACH
- FIGURE 9 CAGR PROJECTIONS, 2024–2029
- FIGURE 10 GROWTH ANALYSIS OF DRIVERS, OPPORTUNITIES, AND CHALLENGES IN CRO SERVICES MARKET
- FIGURE 11 DATA TRIANGULATION METHODOLOGY
- FIGURE 12 CRO SERVICES MARKET, BY TYPE, 2024 VS. 2029 (USD MILLION)
- FIGURE 13 CRO SERVICES MARKET, BY THERAPEUTIC AREA, 2024 VS. 2029 (USD MILLION)
- FIGURE 14 CRO SERVICES MARKET, BY END USER, 2024 VS. 2029 (USD MILLION)
- FIGURE 15 GEOGRAPHICAL SNAPSHOT OF CRO SERVICES MARKET
- FIGURE 16 INCREASING INVESTMENTS IN PHARMACEUTICAL R&D TO DRIVE MARKET
- FIGURE 17 US AND CLINICAL RESEARCH SERVICES TO DOMINATE NORTH AMERICAN CRO SERVICES MARKET IN 2023
- FIGURE 18 CLINICAL RESEARCH SERVICES TO DOMINATE MARKET DURING STUDY PERIOD
- FIGURE 19 PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES ACCOUNTED FOR LARGEST MARKET SHARE IN 2023
- FIGURE 20 ASIA PACIFIC TO REGISTER HIGHEST GROWTH RATE DURING FORECAST PERIOD
- FIGURE 21 DRIVERS, OPPORTUNITIES, AND CHALLENGES: CRO SERVICES MARKET
- FIGURE 22 LARGE PHARMACEUTICAL R&D SPENDING (AS A PERCENTAGE OF SALES), 2013–2022
- FIGURE 23 ACTIVE PHARMACEUTICAL PIPELINE, 2012–2023
- FIGURE 24 BIOSIMILARS APPROVALS AND LAUNCHES, 2015–2022
- FIGURE 25 ONGOING CLINICAL TRIALS BY PHASE AND THERAPEUTIC APPROACH (AS OF APRIL 2023)
- FIGURE 26 GENE & CELL THERAPY DEVELOPERS, CLINICAL TRIALS, AND INVESTMENT, BY REGION, 2022
- FIGURE 27 REVENUE SHIFT AND NEW POCKETS FOR CRO SERVICE PROVIDERS
- FIGURE 28 VALUE CHAIN ANALYSIS: CLINICAL RESEARCH TO ADD MAXIMUM VALUE
- FIGURE 29 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF CRO SERVICES IN PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES
- FIGURE 30 KEY BUYING CRITERIA FOR END USERS
- FIGURE 31 DRUGS IN PIPELINE, BY DEVELOPMENT PHASE, 2022 VS. 2023
- FIGURE 32 NUMBER OF REGISTERED ONCOLOGY CLINICAL TRIALS, 2012–2023 (THOUSAND)
- FIGURE 33 ESTIMATED NUMBER OF INDIVIDUALS WITH DIABETES, 2000–2045 (MILLION)
- FIGURE 34 ANNUAL DRUG APPROVALS, BY CDER (FDA), 2012–2023
- FIGURE 35 R&D SPENDING OF PHARMACEUTICAL MEMBER COMPANIES, 2012–2022
- FIGURE 36 US FDA DE NOVO MEDICAL DEVICE APPROVALS (2012–2023)
- FIGURE 37 NORTH AMERICA: CRO SERVICES MARKET SNAPSHOT
- FIGURE 38 DISTRIBUTION OF R&D COMPANIES, BY COUNTRY/REGION (JANUARY 2022 VS. JANUARY 2023)
- FIGURE 39 ASIA PACIFIC: CRO SERVICES MARKET SNAPSHOT
- FIGURE 40 STRATEGIES ADOPTED BY KEY PLAYERS IN CRO SERVICES MARKET
- FIGURE 41 REVENUE ANALYSIS OF KEY PLAYERS, 2021–2023 (USD BILLION)
- FIGURE 42 MARKET SHARE ANALYSIS OF KEY PLAYERS, 2023
- FIGURE 43 COMPANY EVALUATION MATRIX, 2023
- FIGURE 44 START-UP/SME EVALUATION MATRIX, 2023
- FIGURE 45 IQVIA INC.: COMPANY SNAPSHOT (2022)
- FIGURE 46 ICON PLC: COMPANY SNAPSHOT (2022)
- FIGURE 47 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2022)
- FIGURE 48 LABORATORY CORPORATION OF AMERICA HOLDINGS: COMPANY SNAPSHOT (2022)
- FIGURE 49 SYNEOS HEALTH: COMPANY SNAPSHOT (2022)
- FIGURE 50 WUXI APPTEC: COMPANY SNAPSHOT (2022)
- FIGURE 51 CHARLES RIVER LABORATORIES: COMPANY SNAPSHOT (2022)
- FIGURE 52 PHARMARON: COMPANY SNAPSHOT (2022)
- FIGURE 53 FORTREA, INC.: COMPANY SNAPSHOT (2022)
- FIGURE 54 MEDPACE: COMPANY SNAPSHOT (2022)
- FIGURE 55 SGS SA: COMPANY SNAPSHOT (2022)
- FIGURE 56 FRONTAGE LABS: COMPANY SNAPSHOT (2022)
- FIGURE 57 EUROFINS SCIENTIFIC: COMPANY SNAPSHOT (2022)
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global CRO Services market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
The secondary sources referred to for this research study include publications from public sources, such as the Association of Clinical Research Organizations (ACRO), Clinical Research Society (CRS), Clinical Research Association of Canada (CRAC), Association of International Contract Research Organizations (AICRO), Clinical and Contract Research Association (CCRA), ClinicalTrials.gov website, American Association of Pharmaceutical Scientists (AAPS), Eurostat, Food and Drug Administration (FDA), Pharmaceutical Research and Manufacturers of America (PhRMA), Japan CRO Association, Chinese Association for Laboratory Animal Sciences (CALAS), and Indian Society for Clinical Research (ISCR) among others. Secondary sources also include corporate and regulatory filings (such as annual reports, SEC filings, investor presentations, and financial statements); business magazines and research journals; press releases; business and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the global CRO Services market, which was validated through primary research.
Primary Research
Extensive primary research was conducted after acquiring basic knowledge about the global CRO services market scenario through secondary research. Several primary interviews were conducted with market experts from both the demand side (personnel from pharmaceutical & biopharmaceutical companies, medical device companies, and academic institutes) and supply side (C-level and D-level executives, product managers, and marketing and sales managers of key manufacturers, distributors, and channel partners, among others, from Tier 1 and Tier 2 companies engaged in offering services) across five major regions—North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. Approximately 70% of primary interviews were conducted with supply-side representatives, while demand-side participants accounted for the remaining share. This primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
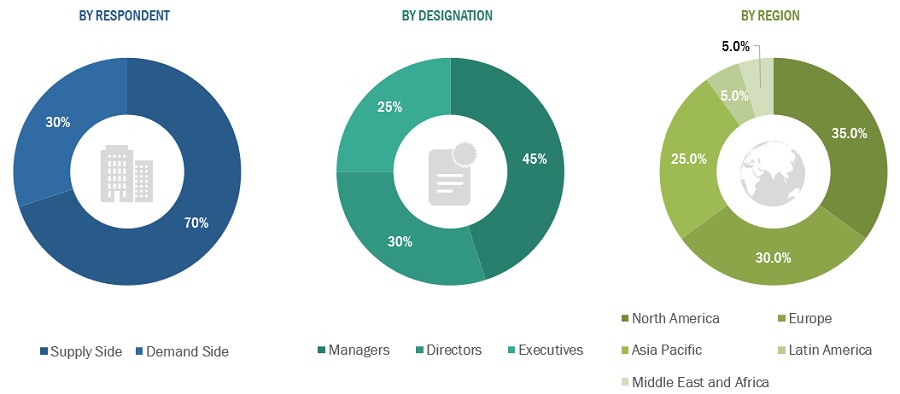
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the CRO services market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Global CRO Services Market Size: Bottom Up Approach
Bottom-up approach was used to estimate and validate the total size of the CRO Services market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and market have been identified through extensive secondary research
- The revenues generated from the CRO Services business of leading players have been determined through primary and secondary research
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
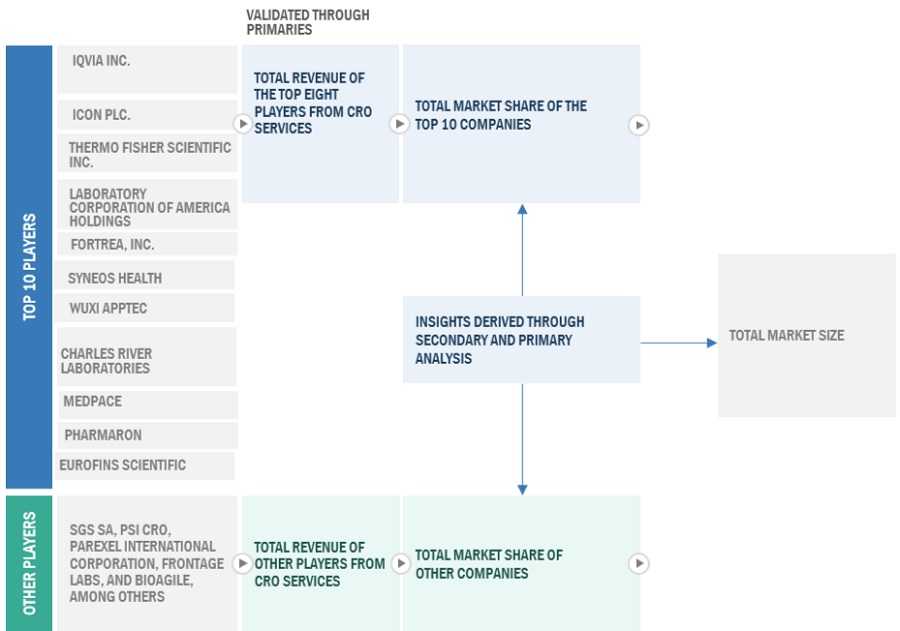
To know about the assumptions considered for the study, Request for Free Sample Report
Global CRO Services Market Size: Top Down Approach

Data Triangulation
After arriving at the overall market size from the market size estimation process, the total market was split into several segments. To complete the overall market engineering process and arrive at the exact statistics for all segments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from the supply side majorly.
Market Definition
CROs are companies that offer research services on a contract basis to various pharmaceutical and biotechnology companies, medical device companies, and academic institutes. These services include clinical research services, early-phase development services, laboratory services, consulting services, and data management services. A sponsor (i.e., a company investigating the safety and efficacy of new drugs, therapies, or medical devices) partners with a CRO on a contractual or project-by-project basis. The CRO provides advice and guidance for planning, designing, and executing clinical trials; it offers a comprehensive and diverse range of services for each phase of the clinical trial.
Key Stakeholders
- Pharmaceutical & biopharmaceutical companies
- Medical device manufacturing companies
- Academic & research institutes
- Venture capitalists & investors
- Market research & consulting firms
- Government associations
- Medical institutions & universities
Report Objectives
- To define, describe, and forecast the contract research organization (CRO) services market based on type, therapeutic area, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (drivers, opportunities, and challenges) along with the current trends
- To strategically analyze micromarkets with respect to their individual growth trends, future prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the revenue of market segments with respect to five major regions: North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa
- To profile the key players and comprehensively analyze their market shares and core competencies
- To track and analyze competitive developments in the CRO services market, such as service launches, agreements, partnerships, collaborations, mergers & acquisitions, and research & development activities
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Portfolio Assessment
- Service Matrix, which gives a detailed comparison of the service portfolios of the top three companies.
Company Information
- Detailed analysis and profiling of additional market players (up to 5).
Geographical Analysis
- A further breakdown of the Rest of Asia Pacific CRO services market into countries
- A further breakdown of the Rest of European CRO services market into countries
- A further breakdown of the Rest of Latin American CRO services market into countries



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Contract Research Organization Services Market
Which are the driving factors of the contract research organization services market?
Who are the major players operating in the contract research organization services market?
Which region will lead the global contract research organization services market?